How many electrons are lost or gained in forming each #Ba^(2+)#?
1 Answer
Two electrons are lost.
Explanation:
The net charge of an atom is always determined by the ratio of protons to electrons.
As you know, the nucleus of an atom contains
- positively charged particles called protons
- neutral particles called neutrons
Notice that the overall charge of a nucleus will always be positive, since you don't have any negative charges to balance the positively charged protons.
Here is where the electrons come into play. As you know, electrons are negatively charged particles that surround the nucleus.
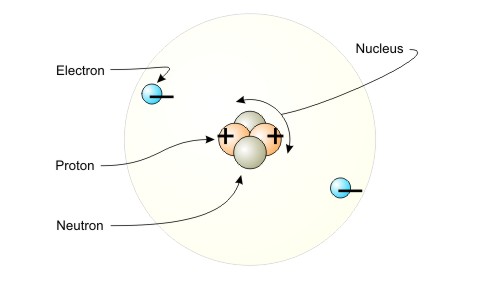
If the number of protons you have in the nucleus is equal to the number of electrons you have surrounding the nucleus, then the atom will be neutral.
Now, an atom that does not have equal numbers of electrons and protons is called an ion. More specifically, it will be called
- a positively charged ion, or cation, if it has more protons than electrons
- a negatively charged ion, or anion, if it has more electrons than protons
In your case, the fact that the atom carries a
More specifically, you're dealing with the
Now, what must happen in order for a neutral barium atom,
Well, the positive charge that it carries tells you that it must have more protons than electrons.
Since you can't add protons and still keep the identity of an element, you can safely state that the atom must have lost electrons.
How many?
Since it carries a
Therefore, you can conclude that a neutral barium atom must lose two electrons in order to gain a

