Where does the maximum electron density occur for 2s and 2p orbitals in hydrogen atom?
1 Answer
For hydrogen, we have to use spherical harmonics, so our dimensions are written as
color(green)(psi_(nlm_l)(r,theta,phi) = R_(nl)(r) Y_(l)^(m_l)(theta, phi))
If you don't understand all of that, that's fine; it was just for context.
To get the maximum electron density, you have to look at probability density curves.
If we plot
The
From this, you can tell that the maximum electron density occurs near
From this similar diagram, we can compare the
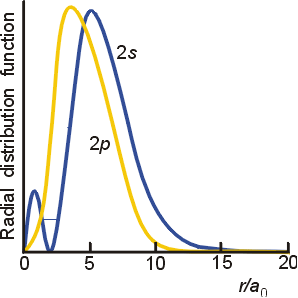
Here, you should see that the
This should make more sense once you realize what the probability density plots of the
2s
2p
"The density of the [dark spots] is proportional to the probability of finding the electron in that region" (McQuarrie, Ch. 6-6).
Basically, start with a radius of 0, and expand your radius of vision outwards from the center of the orbital, and you should be constructing the probability density curves (radial distribution plots).

