Which transition metal can form both a high and low spin complex? #"Zn"^(2+)#, #"Cu"^(2+)#, #"Mn"^(3+)#, #"Ti"^(2+)#
1 Answer
Well, let's see what type of metal each one is, first. The transition metals in Crystal Field Theory are typically classified as
TRANSITION METAL CLASSIFICATIONS
-
The atomic number of zinc is
#30# , so it's on the 10th column in the transition metals. That makes it a#d^10# metal because the electron configuration of#"Zn"^(2+)# is#[Ar]color(red)(4s^0) 3d^10# (take out the two#4s# electrons). -
The atomic number of copper is
#29# , so it's on the 9th column in the transition metals. That makes it a#d^9# metal because the electron configuration of#"Cu"^(2+)# is#[Ar]color(red)(4s^0) 3d^9# (take out the single#4s# electron and the 10th#3d# electron). -
The atomic number of manganese is
#25# , so it's on the 5th column in the transition metals. That makes it a#d^4# metal because the electron configuration of#"Mn"^(3+)# is#[Ar]color(red)(4s^0) 3d^4# (take out the two#4s# electrons and one#3d# electron). -
The atomic number of titanium is
#22# , so it's on the 2nd column in the transition metals. That makes it a#d^2# metal because the electron configuration of#"Ti"^(2+)# is#[Ar]3d^2color(red)(4s^0)# (take out the two#4s# electrons).
Notice how none of these are
CRYSTAL-FIELD SPLITTING DIAGRAMS
All four of these transition metals commonly have coordination numbers of
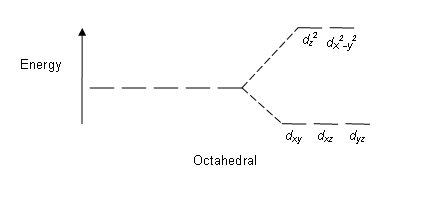
HIGH SPIN VS. LOW SPIN
High spin = fill all five
Low spin = fill lowest-energy
The idea is, which metal(s) have the right number of
1) With zinc, all of its
#d# orbitals are completely filled, so whether a high or low spin octahedral complex, all the orbitals are filled in the exact same configuration.The total spin state turns out to be
#0# (all five sets of#d# electrons are paired).
2) With copper, you can fill the orbitals according to the Aufbau principle, Hund's rule, and the Pauli Exclusion Principle, for both high and low spin octahedral complexes, and you get the same exact configuration.
The total spin state turns out to be
#+"1/2"# (four sets of paired#d# electrons and one unpaired).
3) With manganese, a high spin and a low spin octahedral complex are actually different.
The high-spin octahedral complex has a total spin state of
#+2# (all unpaired#d# electrons), while a low spin octahedral complex has a total spin state of#+1# (one set of paired#d# electrons, two unpaired). BINGO! WE HAVE A WINNER! DING DING DING!
4) With titanium, it only has two
#d# electrons, so it can't form different high and low spin complexes. It doesn't matter because it will never fill the higher-energy orbitals.The total spin state turns out to be
#+1# (two unpaired#d# electrons, no matter what).
Therefore, manganese will form both a high and low spin complex.

