How do you use TTPE to predict bond angles?
1 Answer
I found the usage of "TTPE" on this website:
http://www.scribd.com/doc/21115789/experiment-chem#scribd
The sentence said:
"The real angle bonding for each molecule was determined using TTPE whether it is less or equal with the basic angle."
Whatever it stands for, this nevertheless seems like a systematic way of determining whether the bond angle is the same or smaller than the standard bond angle with no lone pairs of electrons adding coulombic repulsion.
However, it doesn't sound like "TTPE" is supposed to give you exact bond angles.
There is no good way of getting actual bond angles except by advanced computational methods that you don't have to know how to do.
But you CAN predict whether bond angles are equal to or less than the standard bond angle for a compound with no nonbonding electrons in accordance with the VSEPR (valence shell electron pair repulsion) theory.
You can also look up calculated bond angles here:
http://cccbdb.nist.gov/expangle1.asp
As an example, let us compare
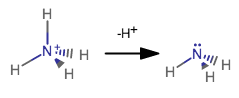
While bonding pairs of electrons are confined to be between atoms, the lone pair takes up more space.
I purposefully crunched together the bond angle for
From the above NIST database reference, if you look up the bond angle for ammonia, you should see:
#/_# #""_("NH"_3) = 106.67^@# compared to the standard
#109.5^@# for tetrahedral molecular geometries that you would expect upon following VSEPR theory.

