How does calcium obey the octet rule when reacting to form compounds?
1 Answer
It loses two electrons from its outermost shell.
Explanation:
Calcium,
In order to have a complete octet, calcium must lose these two outermost electrons, also called valence electrons.
Calcium will react with nonmetals to form ionic compounds.
The electron configuration for a neutral calcium atom looks like this
"Ca: " 1s^2 2s^2 2p^6 3s^2 3p^6 color(red)(4)s^2
After the two outermost electrons are lost, which in a neutral atom occupy the fourth energy level, the calcium cation,
"Ca"^(2+): 1s^2 2s^2 2p^6 color(red)(3)s^2 color(red)(3)p^6
By losing its valence electrons, calcium completes its octet. The outermost shell, which is now the third energy level, holds a total of eight electrons
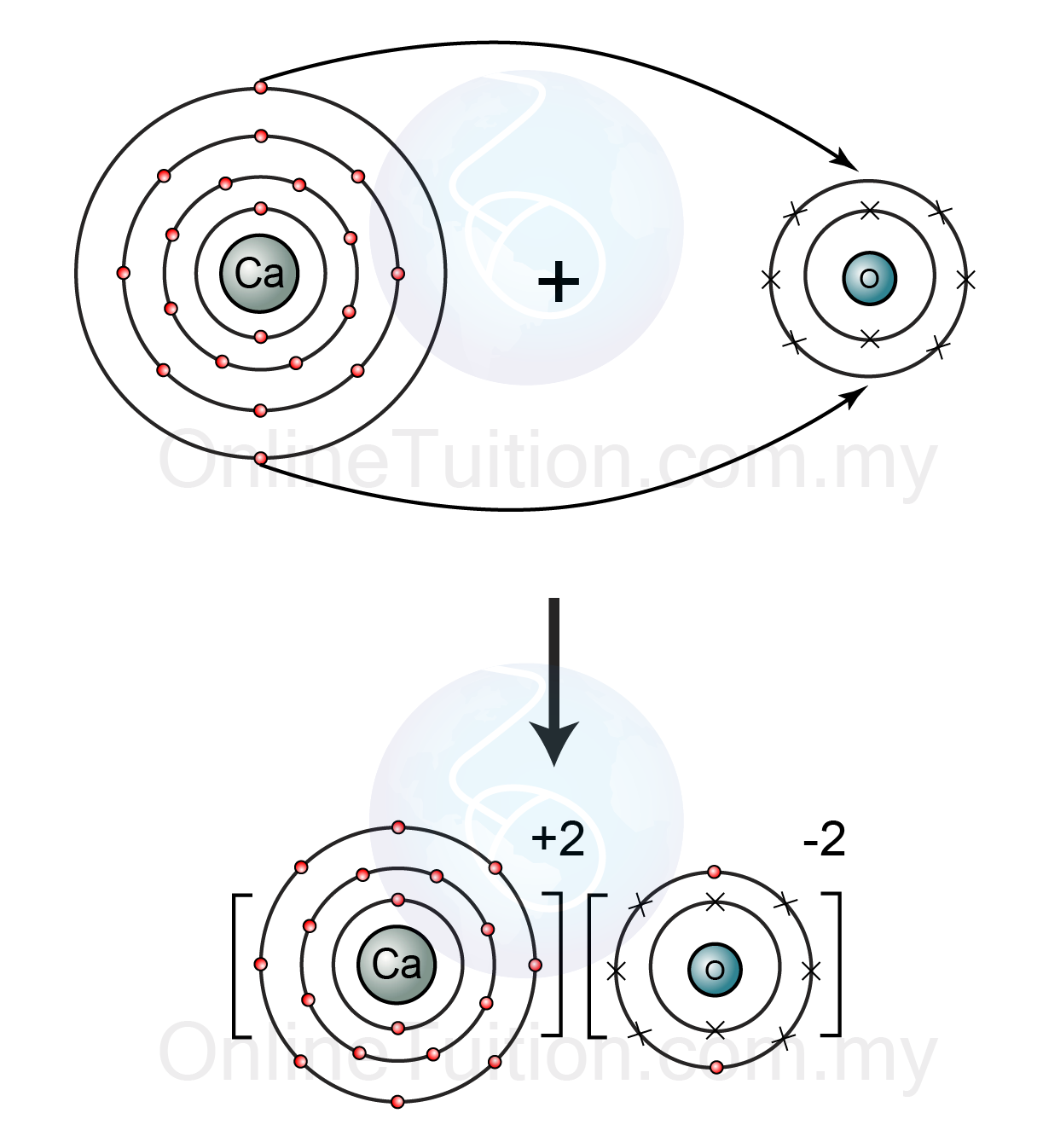
For example, calcium will react with oxygen to form calcium oxide,
Oxygen will pick up these two electrons, forming the

