If in the given structure of a compound there are two chains with an equal number of carbon atoms how do you understand which is the parent chain?
1 Answer
Feb 1, 2016
You choose the chain that has more substituents.
Explanation:
Consider the molecule shown below.
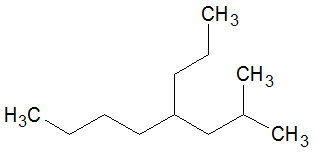
If you count from the left hand end and go up, there are 8 carbons in the chain.
The chain will have an isobutyl group at
If you count from the left hand end and go straight across, there are 8 carbons in the chain.
The chain will have a methyl group at
The second choice has more substituents, so it becomes the parent chain for naming purposes.

