I don't understand the hydrolysis of #Zn(NO_3)_2#: why will the solution be acidic?
This is from the answers volume to my textbook:
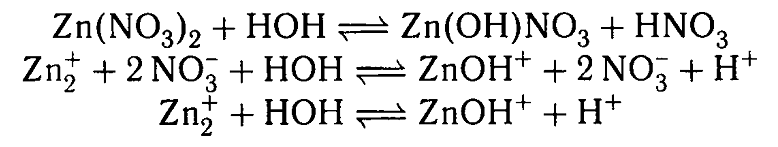
In the middle line, it's clear that we are left with two positively charged ions and two negatively charged ions - I mean 2NO3(-). Why then the solution turns acidic?
Would not the positives and the negatives cancel each other out?
This is from the answers volume to my textbook:
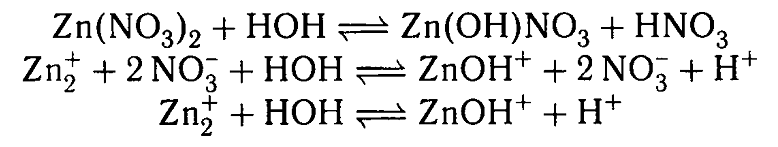
In the middle line, it's clear that we are left with two positively charged ions and two negatively charged ions - I mean 2NO3(-). Why then the solution turns acidic?
Would not the positives and the negatives cancel each other out?
2 Answers
Here's what's going on here.
Explanation:
Before going into anything else, it's important to make sure that you understand what it means to have an acidic solution.
The only indicator to go by when deciding whether or not a solution is acidic, basic, or neutral is the ratio that exists between the hydronium ions,
If you have
#["H"_3"O"^(+)] > ["OH"^(-)] -># the solution is acidic
#["H"_3"O"^(+)] < ["OH"^(-)] -># the solution is basic
#["H"_3"O"^(+)] = ["OH"^(-)] -># the solution is neutral
And that's it. The only way another anion or cation can impact the pH of a solution is if it changes the concentrations of those two ions.
Now, here's a simplified way of looking at that reaction.
Zinc nitrate,
#"Zn"("NO"_3)_text(2(aq]) -> "Zn"_text((aq])^(2+) + 2"NO"_text(3(aq])^(-)#
The nitrate anion is the conjugate base of a strong acid, nitric acid,
On the other hand, the zinc cation is the conjugate acid of a weak base, zinc hydroxide,
#color(blue)("Zn"_text((aq])^(2+) + 4"H"_2"O"_text((l]) rightleftharpoons "Zn"("OH")_text(2(aq]) + 2"H"_3"O"_text((aq])^(+))#
Notice that this reaction produces hydronium ions. This tells you that the balance that exists between hydronium cations and hydroxide anions in a neutral solution will be disrupted, since you now have more hydronium cations that hydroxide anions
This is exactly what your book suggests, but it does so in a slightly different manner.
Here's what's going on there. You can represent zinc nitrate as
#"Zn"^(2+)("NO"_3^(-))("NO"_3^(-))#
and water as
#"H"^(+)("OH"^(-))#
When zinc nitrate is placed in aqueous solution, hydroxide anions coming from water will liberate the nitrate anions in solution. Mind you,
In two stages, this reaction can be written as
#"Zn"^(2+)("NO"_3^(-))("NO"_3^(-)) + "H"^(+)("OH"^(-)) rightleftharpoons "Zn"^(2+)("NO"_3^(-))("OH"^(-)) + "H"^(+) + "NO"_3^(-)#
followed by
#"Zn"^(2+)("NO"_3^(-))("OH"^(-)) + "H"^(+)("OH"^(-)) rightleftharpoons "Zn"^(2+)("OH"^(-))("OH"^(-)) + "H"^(+) + "NO"_3^(-)#
The nitrate anions are spectator ions, which means that you can write the net ionic equation as
#"Zn"^(2+) + "H"^(+)("OH"^(-)) rightleftharpoons overbrace("Zn"^(2+)("OH"^(-)))^(color(purple)("ZnOH"^(+))) + "H"^(+)#
and
#"Zn"^(2+)("OH"^(-)) + "H"^(+)("OH"^(-)) rightleftharpoons overbrace("Zn"^(2+)("OH"^(-))("OH"^(-)))^(color(purple)("Zn"("OH")_2)) + "H"^(+)#
Put all this together to get
#"Zn"^(2+) + "H"^(+)("OH"^(-)) rightleftharpoons color(red)(cancel(color(black)("Zn"^(2+)("OH"^(-))))) + "H"^(+)#
#color(red)(cancel(color(black)("Zn"^(2+)("OH"^(-))))) rightleftharpoons "Zn"^(2+)("OH"^(-))("OH"^(-)) + "H"^(+)#
#color(white)(aaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaa)/color(white)(aaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaaa)#
#color(blue)("Zn"^(2+) + 2["H"^(+)("OH"^(-))] rightleftharpoons "Zn"^(2+)("OH"^(-))("OH"^(-)) + 2"H"^(+))#
Once again, the reaction results in the production of hydronium ions (shown here as
The solution is acidic due to the following:
Explanation:
Aqueous zinc(II) ions are quite acidic as is the case with many metal ions.
In water they are surrounded by 6 water ligands in octahedral symmetery:

Charged metal ions like this have quite a high charge density. This tends to withdraw electron density from the O-H bond thus weakening it.
As a result the ion undergoes hydrolysis:
So you can see that acidic
The ion produced is signified by
Your textbook is very confusing for this. It shows
This is clearly incorrect as charge would not be conserved on either side of the equations. It should read


