What is the hybridization on #CH_4#, #NH_3#, #O_2#, #N_2#, and #H_2O#?
1 Answer
Orbital hybridization is required when one central atom and its surrounding atoms do not have matching orbital symmetries. This can only start occurring for molecules of
In other words, there has to be a need to mix orbitals to create degenerate orbitals that are compatible with the surrounding atoms. This need is not present for binary molecules like
OXYGEN AND NITROGEN
Neither
By convention, the internuclear axis is defined as the
So, what we have is:
- Each oxygen atom bonds to the other using a
#2p_z# atomic orbital and either a#2p_x# or#2p_y# atomic orbital, generating one#sigma# and one#pi# bond, giving a double bond. - Each nitrogen atom bonds to the other using a
#2p_z# atomic orbital as well as both its#2p_x# and#2p_y# atomic orbitals, generating one#sigma# and two#pi# bonds, giving a triple bond.
Neither of them need to hybridize with their
METHANE, AMMONIA, AND WATER
Notice how methane, ammonia, and water all have four electron groups on them. That correlates with a tetrahedral electron geometry.
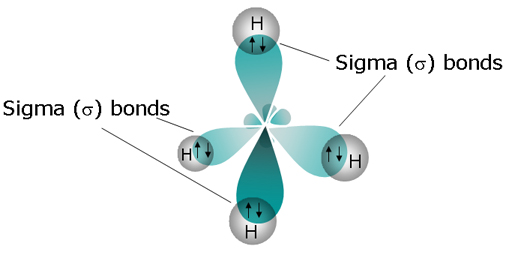
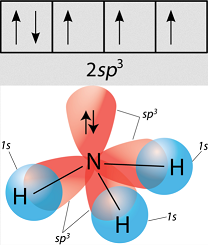
Going off of methane, orbital hybridization is needed because carbon's
Carbon needs to create four identical orbitals that are of the same symmetry as the orbitals contributed by all four hydrogens as a group, so it hybridizes the
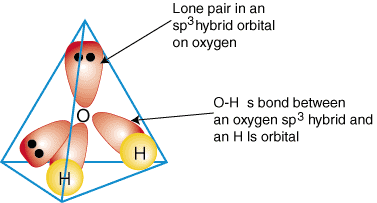
Similarly, ammonia and water have that same issue, and so they hybridize their
As a result, methane, ammonia, and water all have