What is the geometry and hybridization of #"H"_2"S"#? How many #sigma# and #pi# bonds does it have?
1 Answer
Sigma bonds will always be involving orbitals that are symmetric about the internuclear axis, whether it be
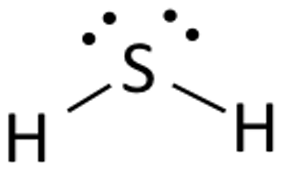
When sulfur could potentially bond with four atoms, it hybridizes its
In other words, sulfur utilizes two identical, compatible
The two others are used to hold one lone pair of electrons each. See how water looks similar?
So, let me ask you this:
- Along which bond is each internuclear axis? (There are two.)
- Are the
#1s# and#sp^3# orbital combinations along the internuclear axis? - If you grab each hydrogen and rotate it about their respective internuclear axes, will you ever see a different
#"H"_2"S"# molecule? - If not, then it is a
#sigma# bond. (The answer is no, so it is a#sigma# bond.)
If you followed all that, you should conclude that there are two
Have you completely accounted for both bonds? (Yes. Hydrogen has no other valence electrons to contribute, and hydrogen normally prefers not to make more than one bond.)
Therefore, there are no

