Would the following molecules react by Sn1 or Sn2 mechanism: 1-methyl-1-bromo-cyclohexane and 2-bromohexane?
1 Answer
First, let me preface by saying that no reaction is necessarily
Some things I could define:
- The compound under each arrow is a solvent.
#"Bu"# is shorthand for#"CH"_3"CH"_2"CH"_2"CH"_2-# , or butyl.- When I wrote substitution, I included both
#S_N1# and#S_N2# under one umbrella, and similarly, when I wrote elimination, I mean both#E1# and#E2# combined.
That aside, let's see.
MAIN POINTS ABOUT SN1 AND SN2
See the following for an overview on these types of reactions:
http://socratic.org/scratchpad/0980681261bdd0bf3e98
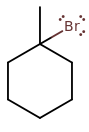
1-BROMO-1-METHYLCYCLOHEXANE
1-bromo-1-methylcyclohexane is a tertiary cyclohaloalkane. It means it has steric hindrance on carbon-1. Therefore, it is more likely to commit to
Why? Because the steric hindrance makes it more difficult to come up from behind and backside-attack. There's too much bulk and obstruction around the target site that it isn't easy for the reactant to get to where it needs to.
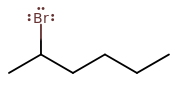
2-BROMOHEXANE
This is where it gets tricky. It is a secondary haloalkane, so it's anyone's guess as to whether
If I had to guess, I would say in general,
As to what extent