Question #eae0f
1 Answer
Explanation:
Start by making sure that you know what you're looking for here.
An element's atomic number,
Every element in the periodic table has an unique atomic number. If two atoms have the same atomic number, then they are isotopes of the same element.
An atom's mass number,
#color(blue)(A = Z + "no. of neutrons")#
Another important thing to remember is that neutral atoms have equal numbers of protons in the nucleus and electrons surrounding the nucleus.
Notice that the problem provides you with an ion of element
In your case, the ion is said to have a
Since the cation is said to contain
#"10 e"^(-) + "3 e"^(-) = "13 e"^(-)#
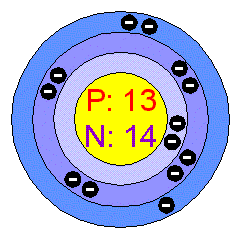
Since a neutral atom has equal number of electrons and protons, you can say that the nucleus of
Therefore, the atomic number of
#Z = 13 -># aluminium
The mass number of the atom will be
#A = Z + 14 = 13 + 14 = color(green)(27)#
You can thus say that you are dealing with the aluminium-27 isotope of aluminium.