What type of reaction is represented by the equation #Zn(s)+H_2SO_4(aq)->ZnSO_4(aq)+H_2(g)#?
2 Answers
The given reaction is a type of Displacement Reaction.
Explanation:
Displacement reaction is a kind of reaction in which a more reactive element displaces a less reactive element from its salt solution or a compound.
The reaction above is also a Single Displacement Reaction between a metal and an acid.
The comparison between reactivity is done using a reactivity series.
Reactivity series is shown below:-
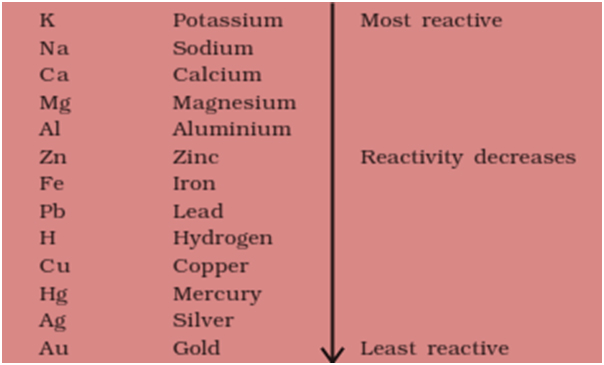
As you see Zinc lies above Hydrogen in this series, so it is more reactive and hence it displaces hydrogen in
It's a redox-reaction.
Explanation:
The zinc is oxidized from
The hydrogen is reduced from
And
Since the sulfate (
The net equation is:

