A compound, #XF_5#, is 42.81% fluorine by mass. What is the element #X#? What is the molecular structure of #XF_5#?
1 Answer
Element
Explanation:
Notice that the problem provides you with the percent composition of fluorine in this unknown compound
This means that you can use a
Once you know that, use the fact that you get
So, a
#m_"X" = "100 g" - "42.81 g" = "57.19 g X"#
Use fluorine's molar mass to get the number of moles of fluorine in the sample
#42.81 color(red)(cancel(color(black)("g"))) * "1 mole F"/(18.9984color(red)(cancel(color(black)("g")))) = "2.2533 moles F"#
Use the
#2.2533 color(red)(cancel(color(black)("moles F"))) * "1 mole X"/(5color(red)(cancel(color(black)("moles F")))) = "0.45066 moles X"#
Well, if
#1color(red)(cancel(color(black)("mole X"))) * "57.19 g"/(0.45066color(red)(cancel(color(black)("moles X")))) = "126.90 g"#
This means that element
A quick look in the periodic table will reveal that element
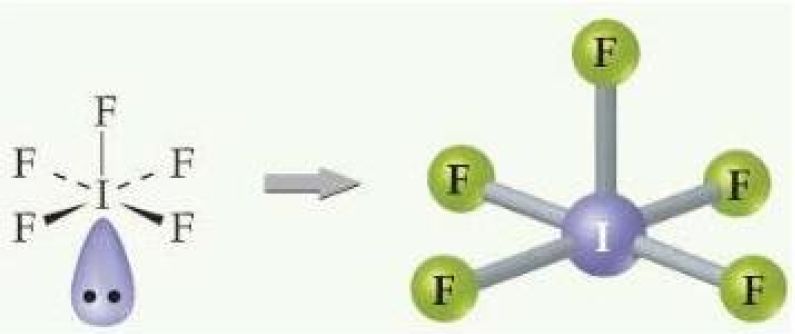
Your unknown compound is iodine pentafluoride,
- five single bonds to each of the five fluorine atoms
- one lone pair of electrons