Question #dbcca
1 Answer
The simple case of an electric dipole is when a positive charge and a negative charge separated by a distance
The electric dipole moment
http://ch301.cm.utexas.edu/section2.php?target=imfs/polar/dipole-moment.html
When you have many charges, a dipole is a separation of the positive charges from the negative ones, you'll have two poles.
Here's an example :
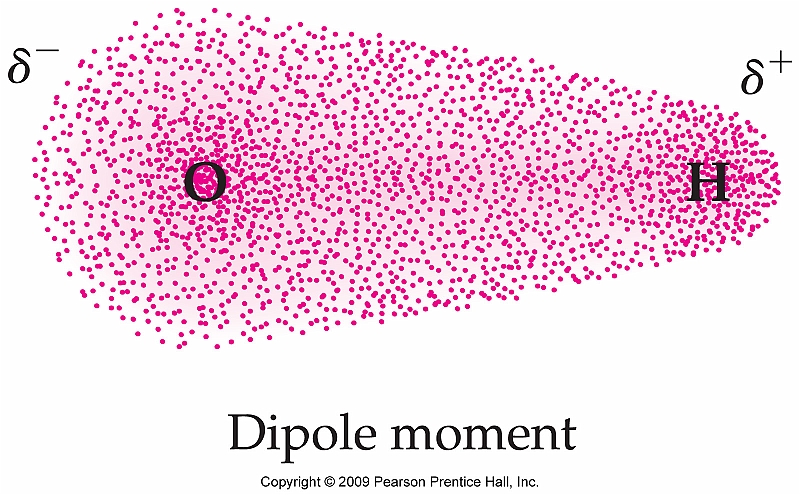 http://stoichiometricbasics.blogspot.com/2012/04/electronegativity-and-gradation-of.html
http://stoichiometricbasics.blogspot.com/2012/04/electronegativity-and-gradation-of.html
The Oxygen is more electronegative so it'll pull the electrons toward it self, the electrons are negatively charged so you'll have a negative charge around the Oxygen
The overall electric dipole moment is the vector addition of all the electric dipole moments :
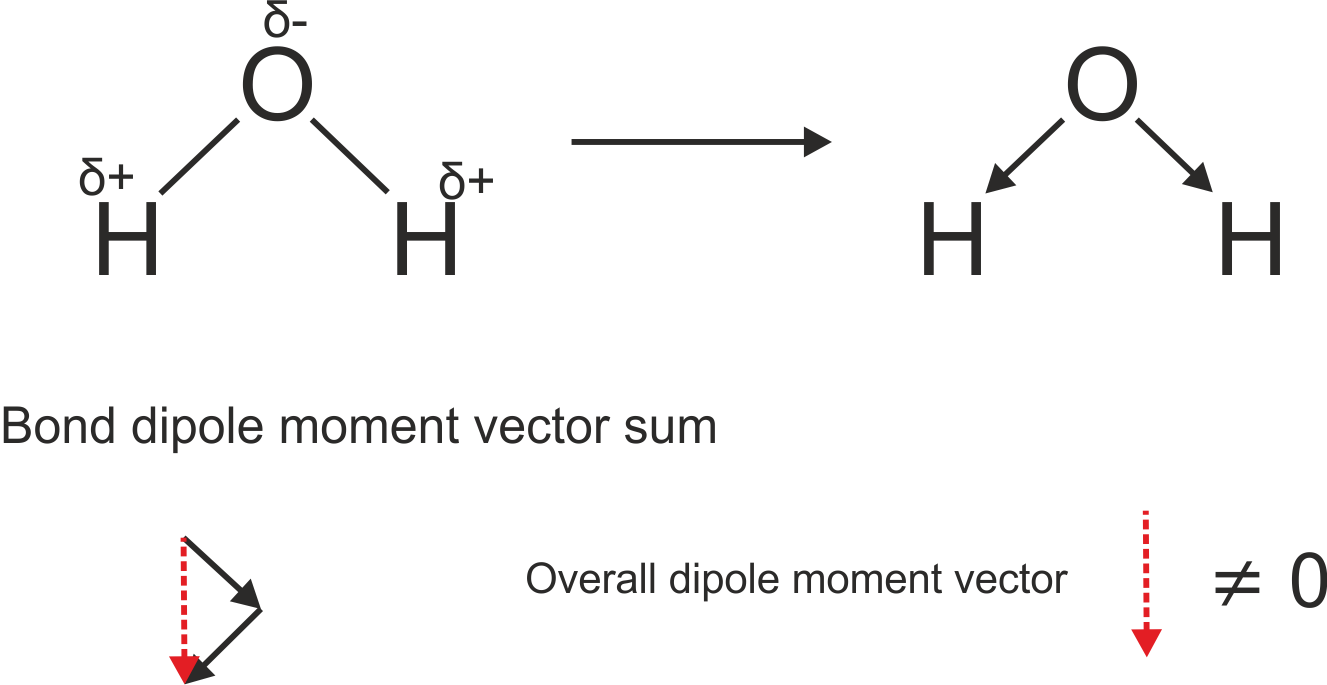 http://www.chemtube3d.com/spectrorotcd0-CE-TEST-ROTATE-ALL.html
http://www.chemtube3d.com/spectrorotcd0-CE-TEST-ROTATE-ALL.html
another example :
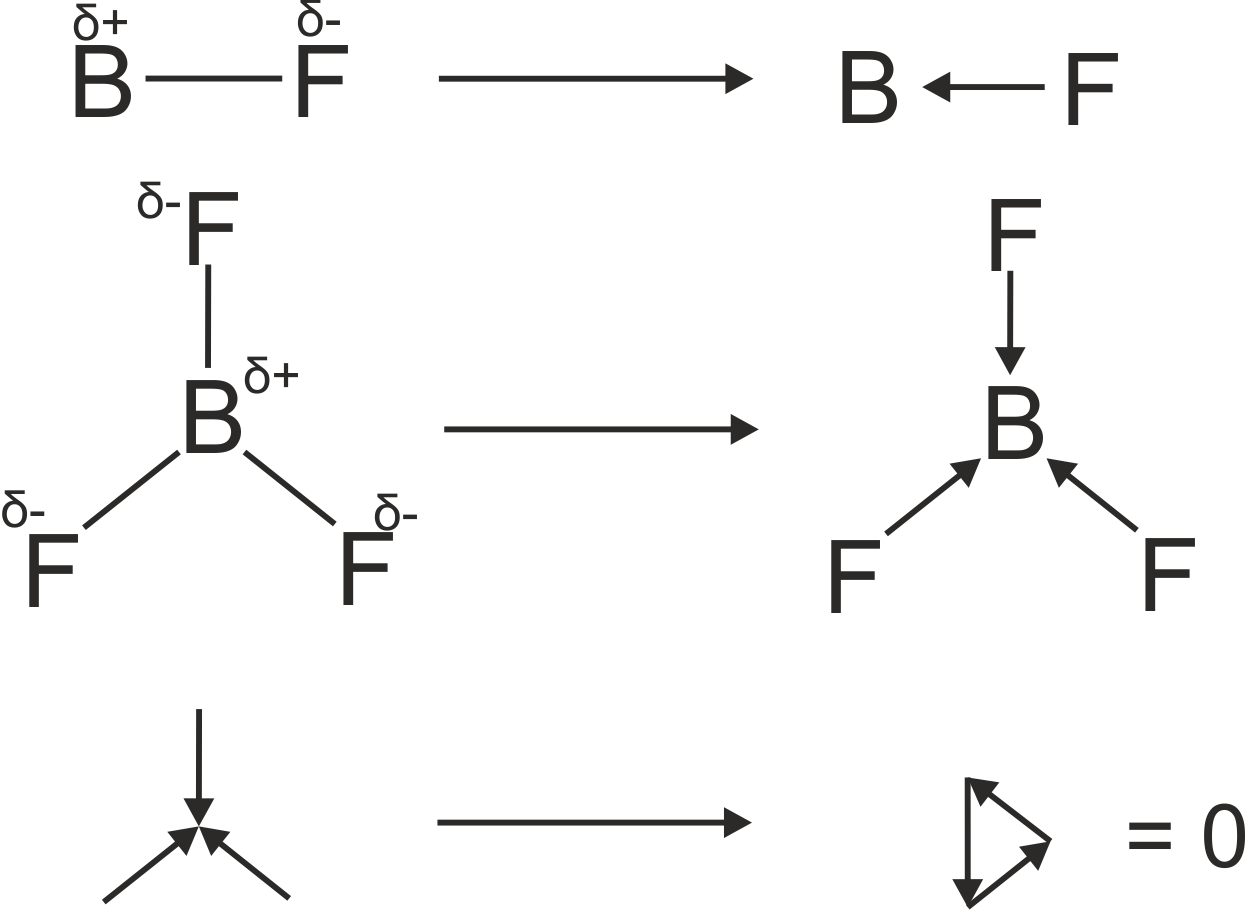 http://www.chemtube3d.com/spectrorotcd0-CE-TEST-ROTATE-ALL.html
http://www.chemtube3d.com/spectrorotcd0-CE-TEST-ROTATE-ALL.html
Sources:
https://en.wikipedia.org/wiki/Electric_dipole_moment
https://en.wikipedia.org/wiki/Dipole
http://chemwiki.ucdavis.edu/Core/Physical_Chemistry/Atomic_Theory/Dipole_Moments

