How does Bohr’s model of the atom explain the line spectrum of hydrogen?
1 Answer
Apr 4, 2016
It is due mainly to the allowed orbits of the electrons and the "jumps" of the electron between them:
Explanation:
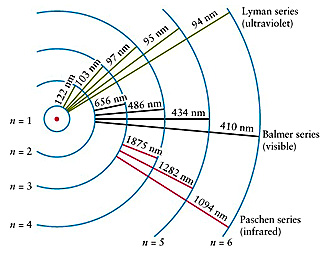
Bohr tells us that the electrons in the Hydrogen atom can only occupy discrete orbits around the nucleus (not at any distance from it but at certain specific, quantized, positions or radial distances each one corresponding to an energetic state of your H atom) where they do not radiate energy.
When the electron moves from one allowed orbit to another it emits or absorbs photons of energy matching exactly the separation between the energies of the given orbits (emission/absorption spectrum).
We see these photons as lines of coloured light (the Balmer Series, for example) in emission or dark lines in absorption.
Hope it helps!

