Which type of bonding can be found in #"As"("C"_6"H"_5)_3#?
#p pi(As)-p pi(C)# #d pi(As)-p pi(C)# #d pi(As)-d pi(C)# #p pi(As)-d pi(C)#
#p pi(As)-p pi(C)# #d pi(As)-p pi(C)# #d pi(As)-d pi(C)# #p pi(As)-d pi(C)#
1 Answer
I'm assuming that you mean a monohapto complex (bound via one carbon to arsenic, not all six). That would be
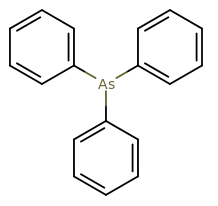
Arsenic has the
Arsenic is utilizing
If we consider the valence atomic orbital energies, according to Miessler et al. Appendix B.9, we have:
#E_(2s)^"C" = -"19.43 eV"# #E_(2p)^"C" = -"10.66 eV"# #E_(4s)^"As" = -"18.94 eV"# #E_(4p)^"As" = -"9.17 eV"#
In the reference, apparently the
We can see that:
- The orbitals of arsenic and carbon are close in energy despite being two quantum levels
#n# apart. - Their
#p# orbitals are sufficiently close in energy (they differ by only#"1.54 eV"# , or#"148.59 kJ/mol"# ), so those can interact favorably.
As
Apparently, that would imply the
I expect the

