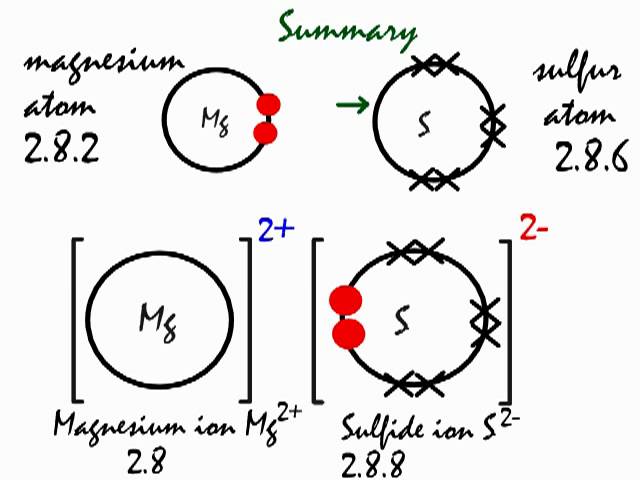
What type of bond do magnesium and sulfur form?
1 Answer
An ionic bond.
Explanation:
Magnesium,
On the other hand, sulfur,
As you know, chemical reactivity is governed by an atom's "desire" to have a stable electron configuration, that is, to have eight electrons in its outermost shell
In this case, magnesium can complete its octet by giving up those two valence electrons, becoming the magnesium cation.
Sulfur, which only needs two electrons to complete it octet, will pick up the two electrons coming from magnesium, becoming the sulfide anion,
The electrostatic force of attraction will then bring the magnesium cations and the sulfur anions together
#color(green)(|bar(ul(color(white)(a/a)color(black)(["Mg"]^(2+)["S"]^(2-) implies "Mg"_2"S"_2 implies "MgS")color(white)(a/a)|)))#