The attachment of a fluoride ion to the boron in #BF_3#, through a coordinate covalent bond, creates the #BF_4-# ion. What is the geometric shape of this ion?
1 Answer
Apr 27, 2016
The
When a ligand (or atom) donates electrons into that
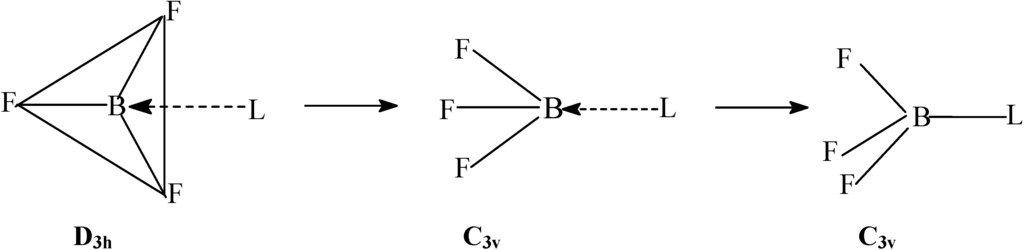
(SIDENOTE: If the atom is also an
You can tell that it's tetrahedral because there are no lone-pair electron groups and there are exactly four electron groups around boron.

