What is a first order half life?
1 Answer
See explanation.
Explanation:
The half-life of a chemical reaction, regardless of its order, is simply the time needed for half of an initial concentration of a reactant to be consumed by the reaction.
Now, a first-order reaction is characterized by the fact that the rate of the reaction depends linearly on the concentration of one reactant.
For a first-order reaction
#"A " -> " products"#
the differential rate law allows you to express the rate of the reaction in terms of the change in the concentration of the reactant (or of a product)
#"rate" = -(d["A"])/dt = k * ["A"]#
The integrated rate law, which establishes a relationship between the change in the concentration of the reactant and time, can be obtained by integrating the differential rate law.
The integrated rate law for a first-order reaction looks like this -- mind you, I will not do the derivation here.
#color(blue)(|bar(ul(color(white)(a/a)color(black)(ln["A"] = ln["A"]_0 - k * t)color(white)(a/a)|)))#
Here
#["A"]# - the concentration of reactant#"A"# after a time#t#
#["A"]_0# - the initial concentration of the reactant#["A"]#
#k# - the rate constant of the reaction
So, the half-life of the reaction,
You can thus say that
#ln(["A"]_0/2) = ln["A"]_0 - k * t_"1/2"#
This can be rearranged to get
#ln(["A"]_0/2) - ln["A"]_0 = - k * t_"1/2"#
#ln(color(red)(cancel(color(black)(["A"]_0)))/2 * 1/color(red)(cancel(color(black)(["A"]_0)))) = -k * t_"1/2"#
#ln(1/2) = - k * t_"1/2"#
You can rewrite this as
#overbrace(ln(1))^(color(blue)(=0)) - ln(2) = - k * t_"1/2"#
#-ln(2) = -k * t_"1/2"#
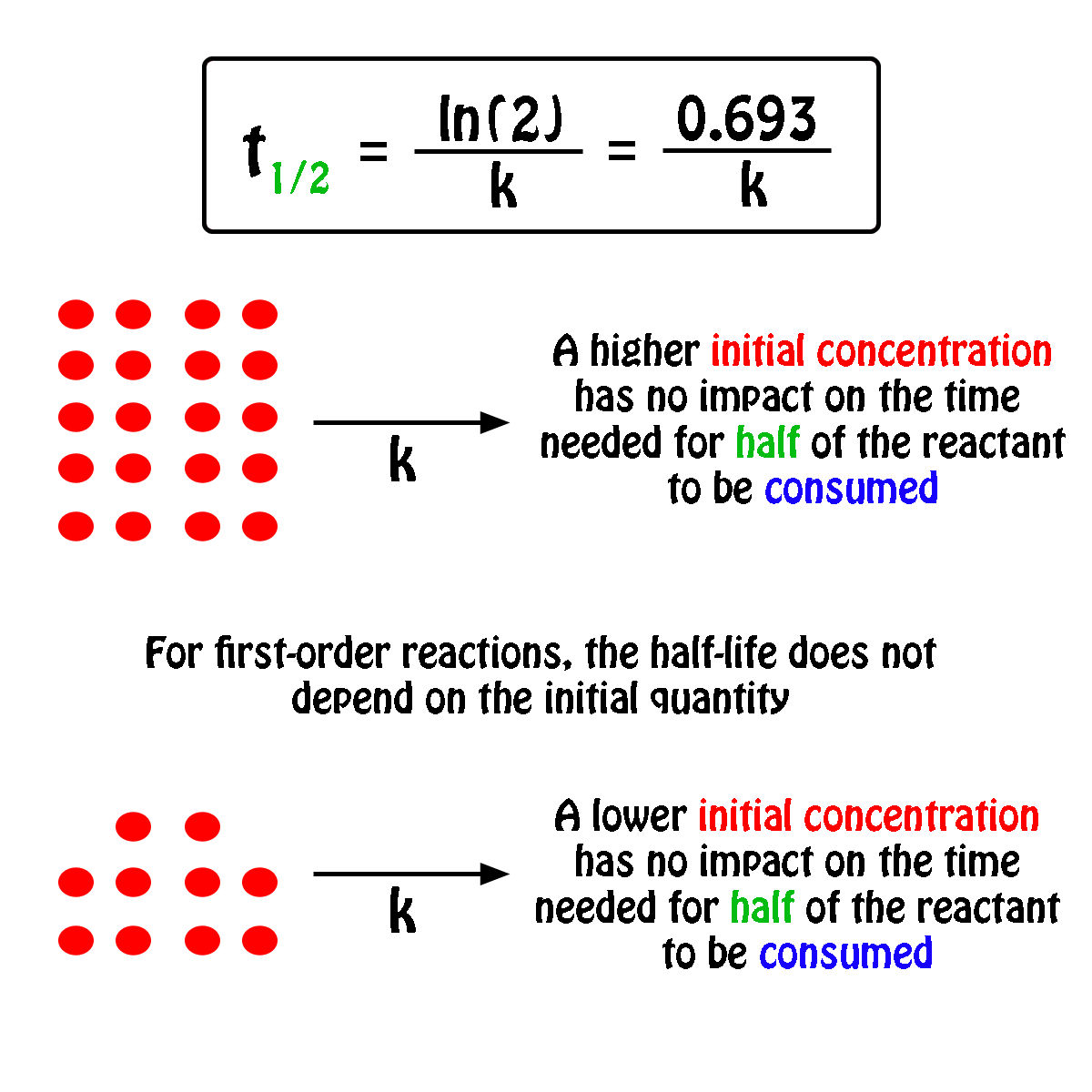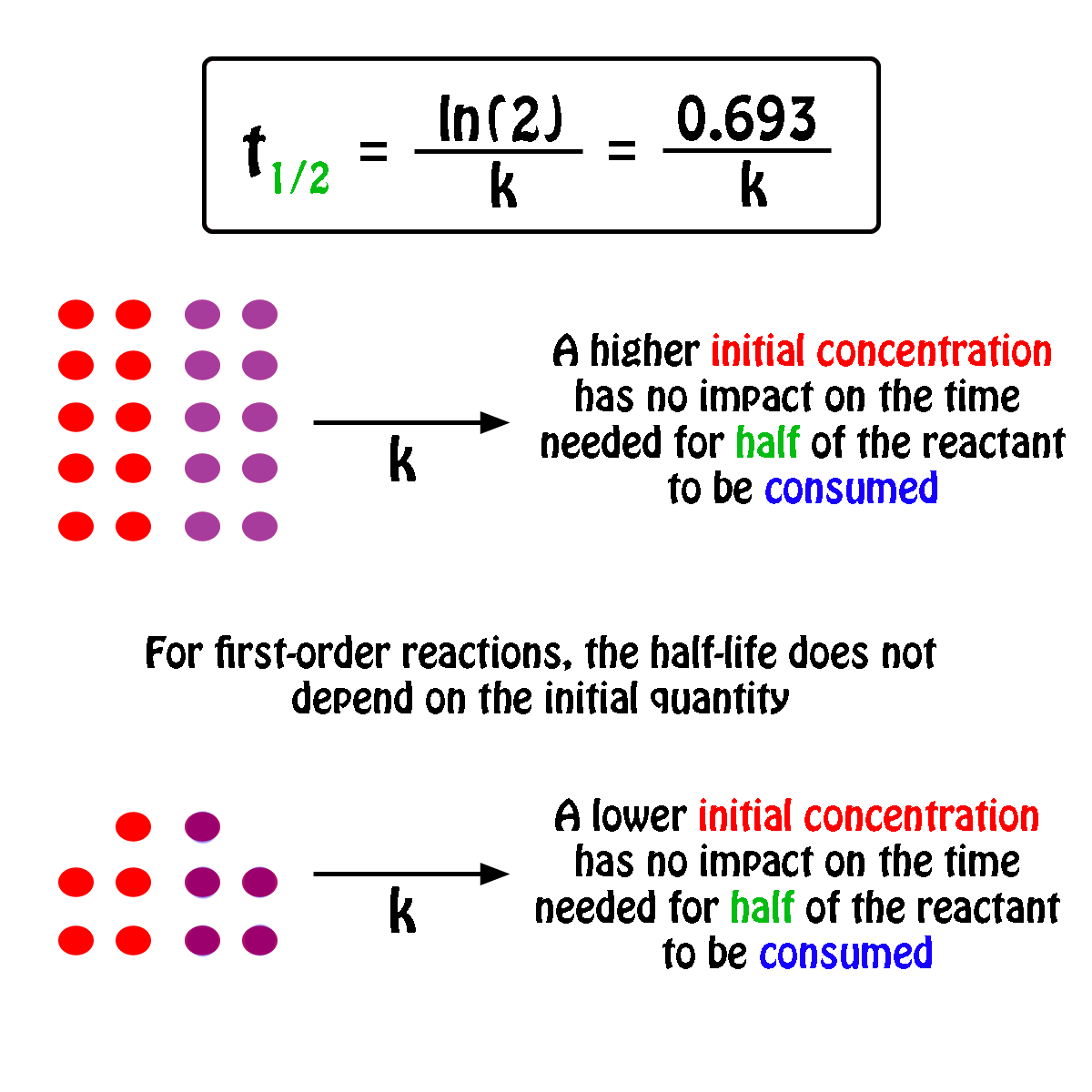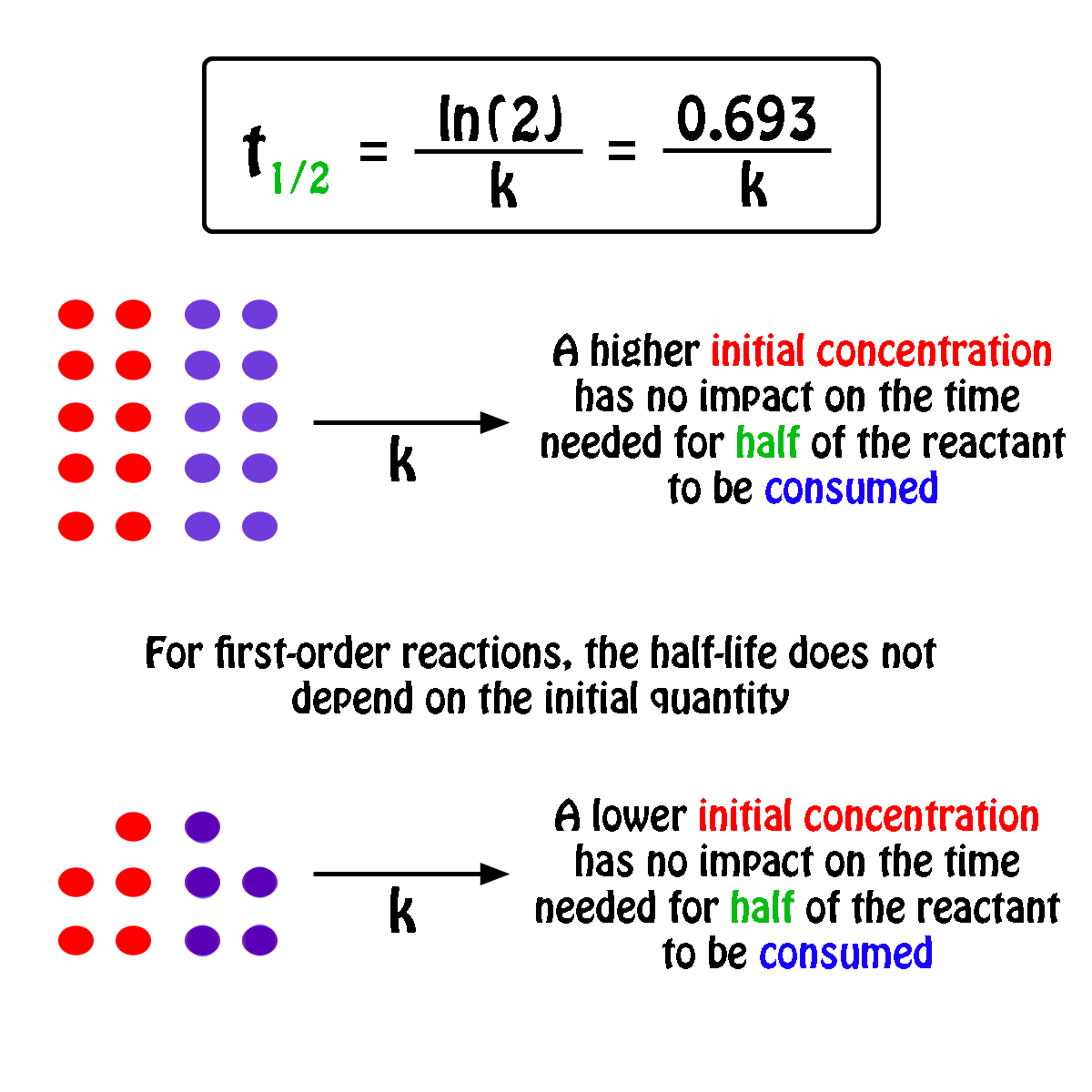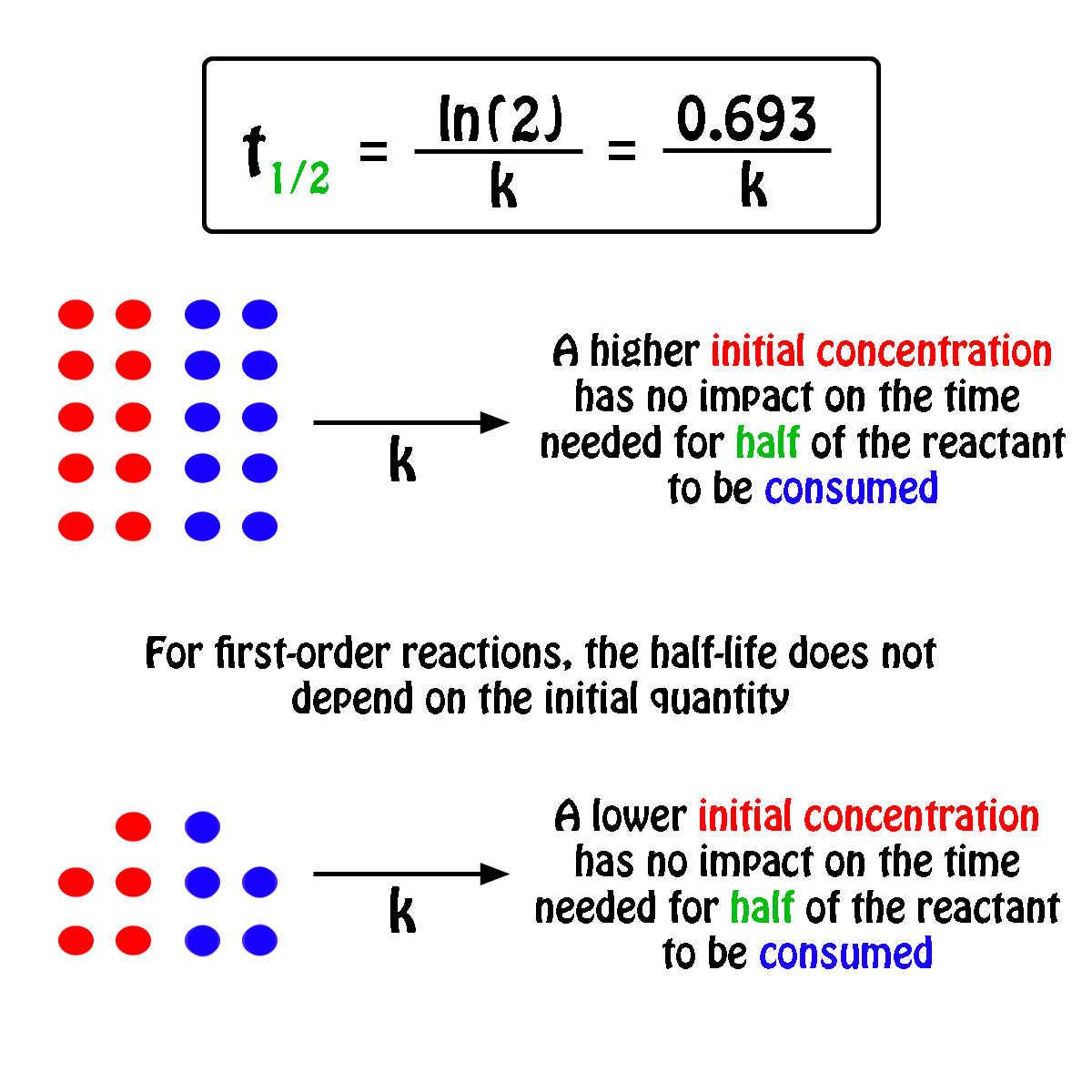
The half-life of a first-order reaction will thus be equal to
#color(green)(|bar(ul(color(white)(a/a)color(black)(t_"1/2" = ln(2)/k)color(white)(a/a)|)))#
An important thing to notice here is that the half-life of a first-order reaction depends exclusively on the rate constant of the reaction.
In other words, the initial concentration of the reactant has no influence on the half-life of the reaction, i.e. the half-life is constant regardless of the concentration of the reactant.