Question #07e8f
1 Answer
The reaction is second-order.
Explanation:
You're dealing with a decomposition reaction of the form
#"AB " -> " products"#
for which the differential rate law can be written as
#"rate" = k * ["AB"]^n#
Here
#k# - the rate constant for the reaction
#n# - the order of the reaction
According to experimental data, you have
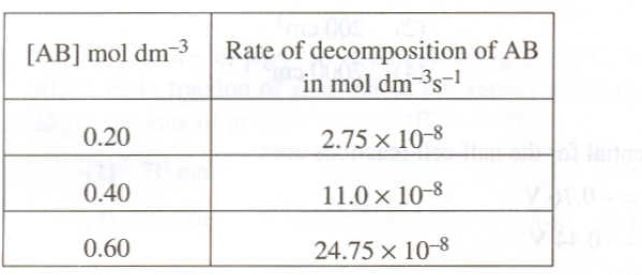
Pick any two entries and plug the data into the differential rate law
#2.75 * 10^(-8)"mol dm"^(-3)"s"^(-1) = k * ("0.20 mol dm"^(-3))^n#
#11.0 * 10^(-8) "mol dm"^(-3)"s"^(-1) = k * ("0.40 mol dm"^(-3)")^n#
You can get rid of the unknown rate constant by dividing these two equations
#(2.73 * color(red)(cancel(color(black)(10^(-8)))) color(red)(cancel(color(black)("mol dm"^(-3)"s"^(-1)))))/(11.0 * color(red)(cancel(color(black)(10^(-8))))color(red)(cancel(color(black)("mol dm"^(-3)"s"^(-1))))) = color(red)(cancel(color(black)(k)))/color(red)(cancel(color(black)(k))) * (0.20)^n/(0.40)^n color(red)(cancel(color(black)(("mol dm"^(-3))^n)))/color(red)(cancel(color(black)(("mol dm"^(-3))^n)))#
#2.75/11.0 = (0.20/0.40)^n#
This is equivalent to
#ln(2.75/11.0) = ln[ (0.20/0.40)^n]#
Solve for
#ln(1/4) = n * ln(1/2)#
#ln[(1/2)^2] = n * ln(1/2)#
#2 * color(red)(cancel(color(black)(ln(1/2)))) = n * color(red)(cancel(color(black)(ln(1/2))))#
Therefore,
#n = 2#
and your decomposition reaction is second-order.
SIDE NOTE The order of the reaction must come out the same regardless of what data you chose to use in the calculations.
I recommend using all the possible combinations of data sets to see that this is the case.
#ln(24.75/11.0) = n * ln(0.60/0.40)#
#ln(2.75/24.75) = n * ln(0.20/0.60)#

