Question #7b0f6
1 Answer
Explanation:
Phosphorus,
-
the atomic number of the element
#-># this tells you the total number of electrons present in a neutral phosphorus atom. -
the group in which phosphorus is located in the periodic table
#-># for main-group elements, the group number tells you the number of bonding electrons present in a neutral atom
The idea is that an atom's electrons can basically be split into two groups bonding and non-bonding.
Bonding electrons, also known as valence electrons, are the electrons located in the outermost shell of the atom. These are the electrons used for chemical reactions.
On the other hand, non-bonding electrons, also known as core electrons, are located in the inner shells of the atom and are not used for chemical reactions.
You can thus say that
#color(blue)(|bar(ul(color(white)(a/a)"total no. of e"^(-) = "no. of bonding e"^(-) + "no. of non-bonding e"^(-)color(white)(a/a)|)))#
So a, neutral atom has equal numbers of protons in the nucleus and electrons surrounding the nucleus. As you know, the number of protons present in the nucleus is given by the atomic number.
Grab a periodic table and look for phosphorus. You'll find it in period 3, group 15. Its atomic number is equal to
This means that a neutral phosphorus atom has a total of
Since it's located in period 15, you know that phosphorus has a total of
#color(blue)(|bar(ul(color(white)(a/a)"group number" = "no. of valence e"^(-)color(white)(a/a)|)))#
So, phosphorus has
#"no. of non-bonding e"^(-) = "15 e"^(-) - "5 e"^(-) = color(green)(|bar(ul(color(white)(a/a)color(black)("10 e"^(-))color(white)(a/a)|)))#
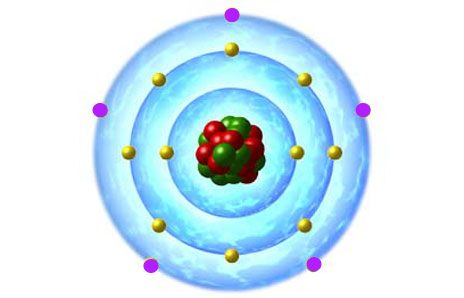
Here's how an atomic structure diagram would look for a neutral phosphorus atom.
The valence electrons, shown here in purple, are located on the outermost energy shell. The non-bonding electrons, shown here in yellow, are located on the two inner shells.