Question #66e6d
1 Answer
The answer is (3)
Explanation:
The idea here is that the temperature coefficient, which you'll sometimes see referred to as the
Simply put, the
The equation that allows you to calculate the
#color(blue)(|bar(ul(color(white)(a/a)Q_(10) = ("rate"_2/"rate"_1)^(10/(T_2 - T_1))color(white)(a/a)|)))#
Here
Now, a
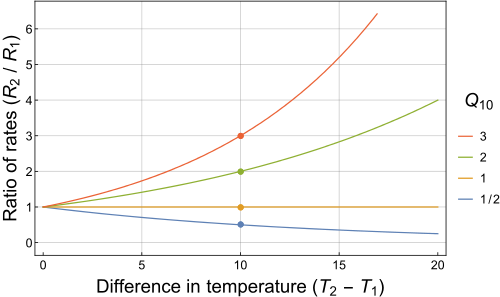
It's important to notice that because you're dealing with change in temperature, you can skip the conversion to Kelvin.
That is the case because an increase by
So in the case of a
#T_2 - T_1 = "10 K"#
and
#Q_(10) = ("rate"_2/"rate"_1)^(10/10) = "rate"_2/"rate"_1#
In your case,
#3 = "rate"_2/"rate"_1 implies color(purple)(|bar(ul(color(white)(a/a)color(black)("rate"_2 = 3 xx "rate"_1)color(white)(a/a)|)))#
For your reaction, the temperature goes from
#DeltaT = 65^@"C" - 35^@"C" = 30^@"C"#
So, if the rate of the reaction increases by factor of
#30^@"C" = 3 xx 10^@"C"#
increase will cause the rate to go up by
#"rate"_2 = overbrace(color(white)(aaa)3color(white)(aaa))^(color(darkgreen)("for a 10"^@"C increase")) xx overbrace(color(white)(aaa)3color(white)(aaa))^(color(darkgreen)("for a 10"^@"C increase")) xx overbrace(color(white)(aaa)3color(white)(aaa))^(color(darkgreen)("for a 10"^@"C increase")) xx "rate"_1#
#"rate"_2 = 27 xx "rate"_1#
Therefore, the rate of the reaction will increase by a factor of

