What is a set of four quantum numbers that could represent the last electron added (using the Aufbau principle) to the Cl atom?
1 Answer
Here's what I got.
Explanation:
Your starting point here will be the electron configuration of a neutral chlorine atom.
Chlorine is located in period 3, group 17 of the periodic table and has an atomic number equal to
The electron configuration of a chlorine atom looks like this
#"Cl: " 1s^2 2s^2 2p^6 3s^2 3p^5#
Now, the last subshell to be filled with electrons, which is also the highest in energy, is the
As you can see from the electron configuration, this subshell contains a total of
As you know, we can use a set of four quantum numbers to describe the location and spin of an electron in an atom
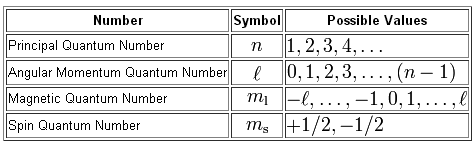
Let's start with the principal quantum number,
#n=3 -># the third energy level
The angular momentum quantum number,
#l=1 -> # the p-subshell
Now, this is where things can get a little tricky. According to Hund's Rule, every orbital in a given subshell must be occupied with
You know that the
After this happens, the second-to-last electron will occupy the
Finally, the last electron to be added will be placed in the
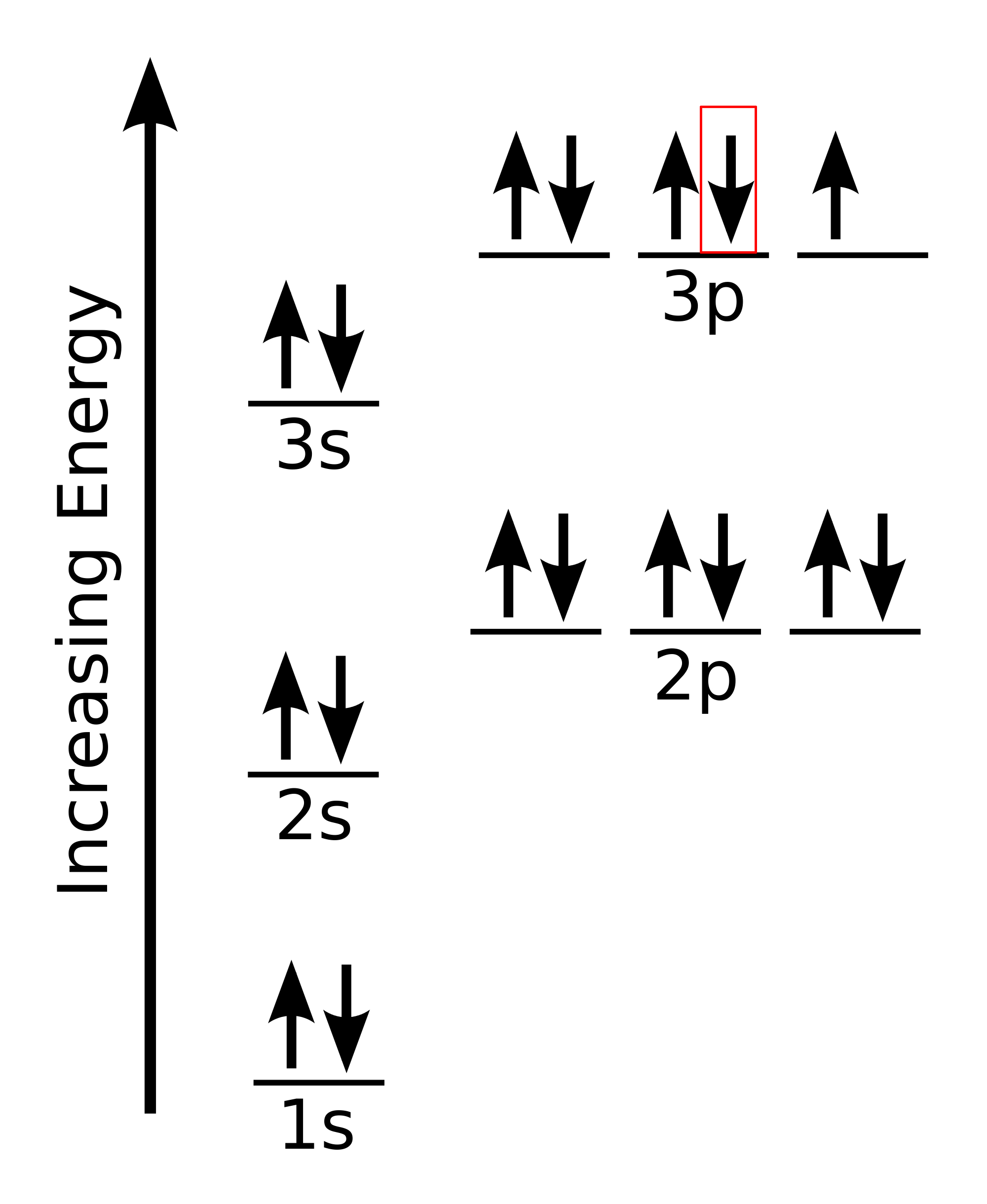
So, the magnetic quantum number,
#m_l = -1 -># the#3p_x# orbital#m_l = color(white)(-)0 -># the#3p_z# orbital#m_l = +1 -># the#3p_y# orbital
In this case, you would have
#m_l = 0 -># the#3p_z# orbital
Finally, the spin quantum number,
#m_s = -1/2 -># a spin-down electron
Therefore, a possible quantum number set for the last electron added to a chlorine atom is
#color(green)(|bar(ul(color(white)(a/a)color(black)(n=3, l=1, m_l = 0, m_s = -1/2)color(white)(a/a)|)))#

