Why does cyclohexene have a lower melting point than cylcohexane?
1 Answer
Jul 6, 2016
Here's my explanation.
Explanation:
Melting points depend, not only on the strength of intermolecular forces, but also on the ability of the molecules to pack into a crystalline array.
This, in turn, depends on their shape.
Cyclohexane has a high degree of symmetry, and this enables the molecules to pack nicely into a crystalline structure with strong London dispersion forces between them..
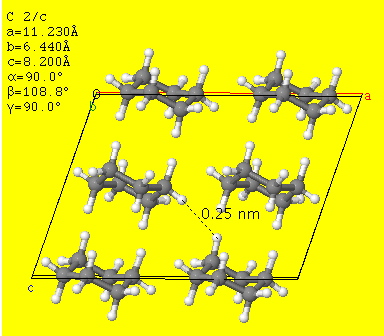
Cyclohexene exists in a half-chair conformation.

It has less symmetry than hexane, so it does not pack as well into a crystal structure.
Thus, cyclohexene has a much lower melting point than cyclohexane (-103.5 °C vs. 6.5 °C).

