Question #04115
1 Answer
Because oxygen is more electronegative than hydrogen.
Explanation:
A water molecule is made up of
- one oxygen atom,
# 1 xx "O"# - two hydrogen atoms,
#2 xx "H"#
The oxygen atom is bonded to each of the two hydrogen atoms via a single bond, which as you know is a chemical bond in which two electrons called bonding electrons are being shared between the two atoms.
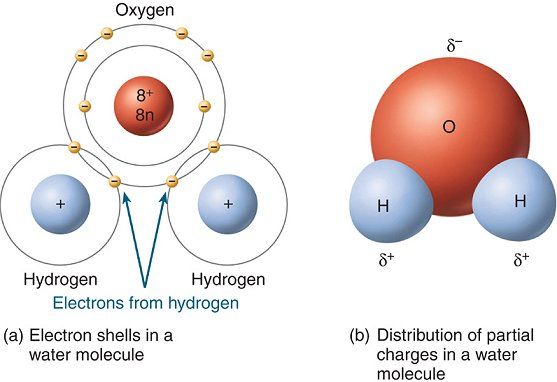
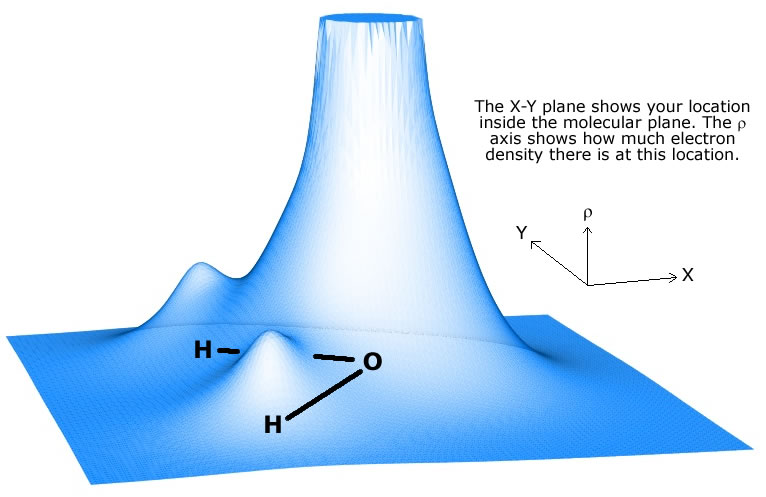
Now, a covalent bond that exists between two atoms is considered polar if one of the two atoms is more electronegative than the other.
Electronegativity is simply a measure of how a bonded atom attracts the bonding electrons. The more electronegative the atom, the more it will attract the bonding electrons towards itself.
When that happens, the more electronegative atom develops a partial negative charge,
This is what causes the bond to become polar. A polar covalent bond is simply a bond in which the bonding electrons are not shared equally between the two atoms.
This is what happens in water's case. Oxygen is much more electronegative than hydrogen, to it will attract the electron density, i.e. the bonding electrons, more than hydrogen.