If #32 L# of a gas at room temperature exerts a pressure of #8 kPa# on its container, what pressure will the gas exert if the container's volume changes to #8 L#?
1 Answer
Jul 11, 2016
The pressure is 32kPa.
Explanation:
Let's identify our known and unknown variables.
The first volume we have is
We can obtain the answer using Boyle's Law which shows that there is an inverse relationship between pressure and volume as long as the temperature and number of moles remain constant.
We will use this equation:
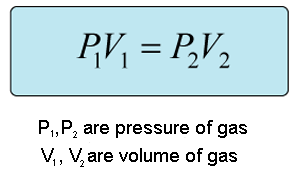
All we have to do is rearrange the equation to solve for
We do this by dividing both sides by
Now all we have to do is plug in the given values:

