What is the mass of a piece of metal that raises the volume of water in a graduated cylinder from #"15.0 cm"^3# to #"70.0 cm"^3# ? the density of iron is #"5.1 g/cm"^3#
1 Answer
Explanation:
The idea here is that the volume of the metal will cause the volume of the water to increase from its initial value of
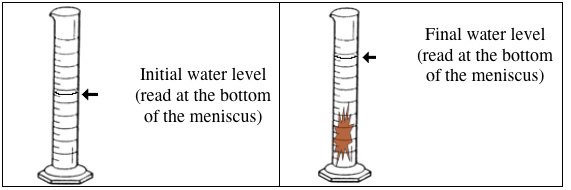
In other words, the difference between the final volume of the water, i.e. the volume of the water + metal, and the initial volume of the water will give you the volume of the metal
#V_"metal" = "70.0 cm"^3 - "15.0 cm"^3 = "55.0 cm"^3#
Now, the problem tells you the metal has a density of
In your case,
#55.0 color(red)(cancel(color(black)("cm"^3))) * overbrace("5.1 g"/(1color(red)(cancel(color(black)("cm"^3)))))^(color(purple)("given density")) = color(green)(|bar(ul(color(white)(a/a)color(black)("281 g")color(white)(a/a)|)))#
I'll leave the answer rounded to three sig figs.

