What is an isothermal process with an example?
1 Answer
An isothermal process is one for which
Explanation:
Consider a phase change under constant temperature, as induced by a pressure change. Consulting any phase diagram will show you that multiple phases, or even allotropes, of a species may exist at a given temperature
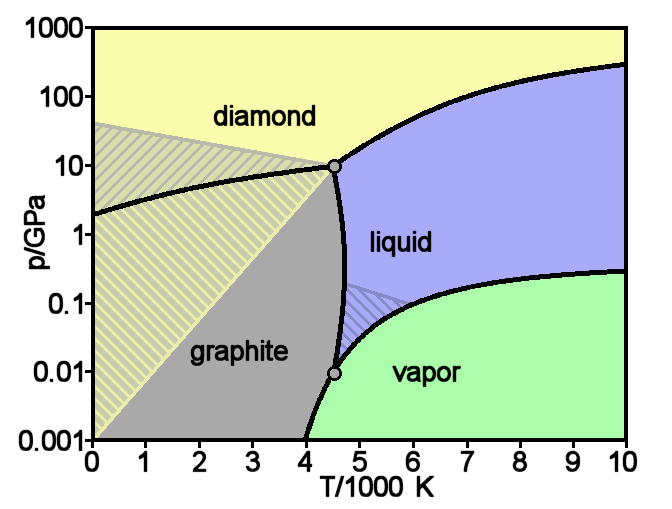
This phase diagram demonstrates a triple point - conditions that cause a sample to exhibit three of its states of matter - at a pressure of
As a contrasting side note, a distinction can be made between an isothermal process and an adiabatic one. In the case of the latter heat cannot be transferred to or from the surroundings, although

