To what pressure would you have to compress 48.0 L of oxygen gas at 99.3 kPa in order to reduce its volume to 16.0 L?
1 Answer
Aug 11, 2016
Explanation:
The answer can be determined by using Boyle's Law:
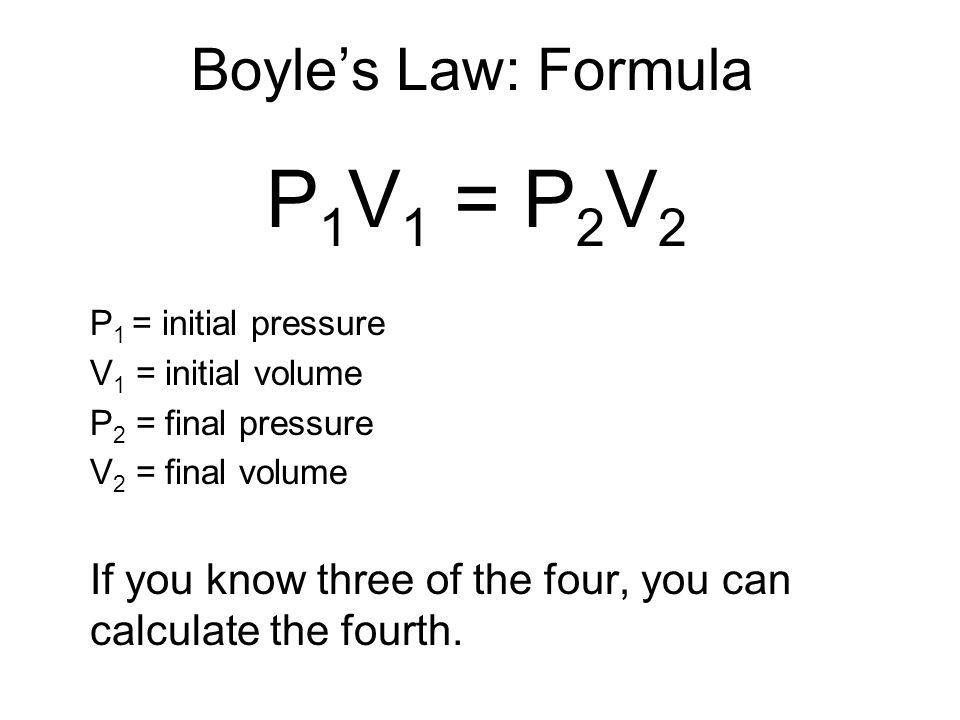
Let's identify our known and unknown variables.
Rearrange the equation to solve for the final pressure by dividing both sides by
Plug in your given values to obtain the final pressure:

