What is the pH of a .073 M #HCl# solution?
1 Answer
Aug 13, 2016
The solution has a pH of 1.14
Explanation:
Since HCl is a strong acid, meaning that it completely ionizes when dissolved in water, the pH can be obtained directly from the concentration using the formula below:
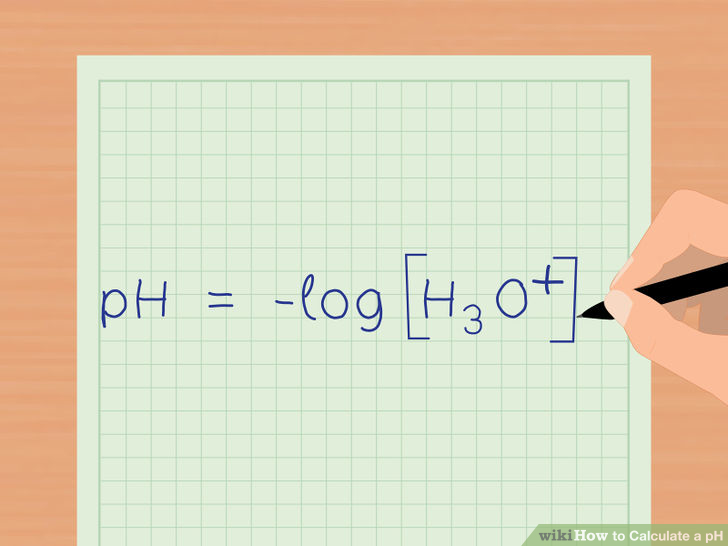
Take the -logarithm of the concentration of hydronium ions that are in the solution:

