A block of aluminum occupies a volume of 15.0 mL and weighs 40.5 g. What is it density?
1 Answer
Aug 18, 2016
Explanation:
To calculate the density, we have to use the formula below:
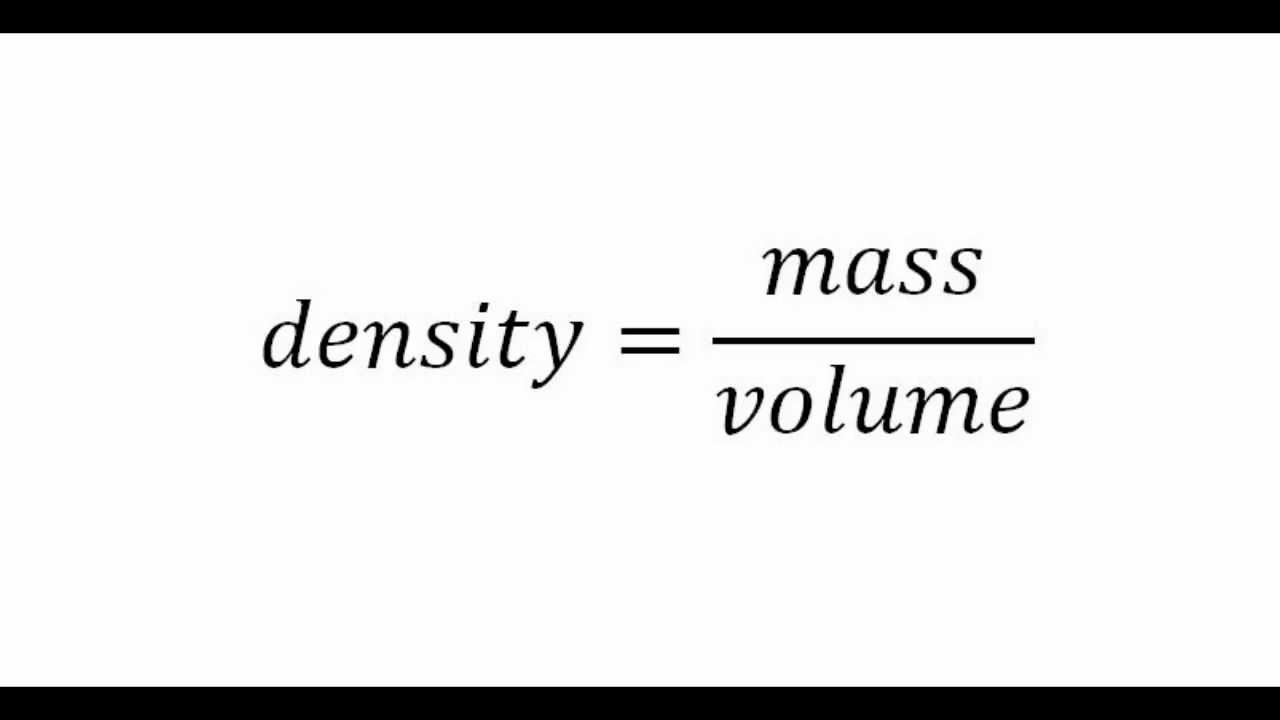
- Usually, density will have units of
-
The mass has units of grams,
#g# . -
The volume will have units of
#mL# or#cm^3#
We are given the mass and the volume, both of which have good units, so all we have to do is plug the given values into the equation:
Thus, the block of aluminum has a density of 2.70 g/mL.

