What are the main energy levels where the valence electrons belong called?
1 Answer
The valence electrons are located in the valence shell.
Explanation:
The valence electrons for the representative (main) group of elements are found in the outermost (highest energy) s and p sublevels. They are often together called the valence shell. Electron configurations can make it easy to see the valence shells for the atoms of the elements.
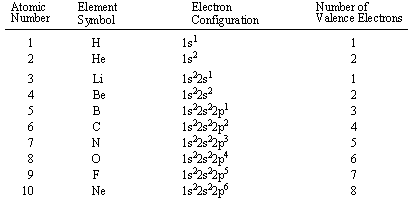
Hydrogen (H) and helium (He) have a valence shell containing one and two electrons respectively. They make up the first period (row) of the periodic table. Their valence electron/s are in the first energy level
The period number corresponds to the main energy level that is filling with electrons, which make up the valence shell.
The elements lithium (Li) through neon (Ne) make up the second period of the periodic table. Their valence electrons are in the 2nd energy level
In the diagram above, compare the number of valence electrons for each element with the number of electrons in the highest energy level.

