Question #9b3cf
1 Answer
Here's what I got.
Explanation:
The first thing to do here is to write the electron configuration of a neutral aluminium atom.
Aluminium is located in period 3, group 13 of the Periodic Table of Elements and has a total of
The electron configuration of a neutral aluminium atom looks like this
#"Al: " 1s^2 2s^2 2p^6 3s^2 color(blue)(3)p^1#
As you can see, the last electron present in an aluminium atom is located in a
Now, we need to find the values of the four quantum numbers used to describe the position and spin of an electron inside an atom.
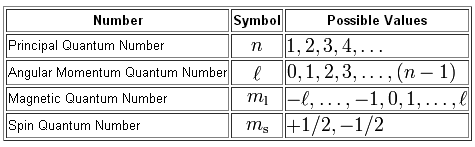
In your case, the principal quantum number,
The angular momentum quantum number,
#l=0 -># designates the s subshell#l=1 -># designates the p subshell#l=2 -># designates the d subshell
and so on. The magnetic quantum number,
#m_l = -1 -># the#3p_x# orbital#m_l = color(white)(-)0-># the#3p_z# orbital#m_l = color(white)(-)1 -># the#3p_y# orbital
Because in the case of an aluminium atom the p subshell contains a single electron, you can pretty much pick any of these three values for
Let's say that we have
Finally, the spin quantum number,
Since your electron is alone in the
Therefore, you can say that a valid set of quantum numbers that describe the last electron added to an aluminium atom could be
#n=3, l=1, m_l = 0, m_s = +1/2# This describes an electron located on the third energy level, in the 3p-subshell, in the
#3p_z# orbital, that has spin-up
You could also have, for example
#n=3, l=1, m_l = -1, m_s = +1/2# This describes an electron located on the third energy level, in the 3p-subshell, in the
#3p_x# orbital, that has spin-up
#n=3, l=1, m_l = 1, m_s = +1/2# This describes an electron located on the third energy level, in the 3p-subshell, in the
#3p_y# orbital, that has spin-up
Here is a video with more explanation of quantum numbers.


