Question #cbc5e
1 Answer
Explanation:
Right from the start, you should be able to look at the information provided by the problem and be able to say that the new volume will definitely be smaller than
That is the case because pressure and volume have an inverse relationship when temperature and number of moles are *kept constant, as given by Boyle's Law.
Simply put, when you keep the temperature and the number of moles of gas constant, you can increase its volume by decreasing its pressure and decrease its volume by increasing its pressure.
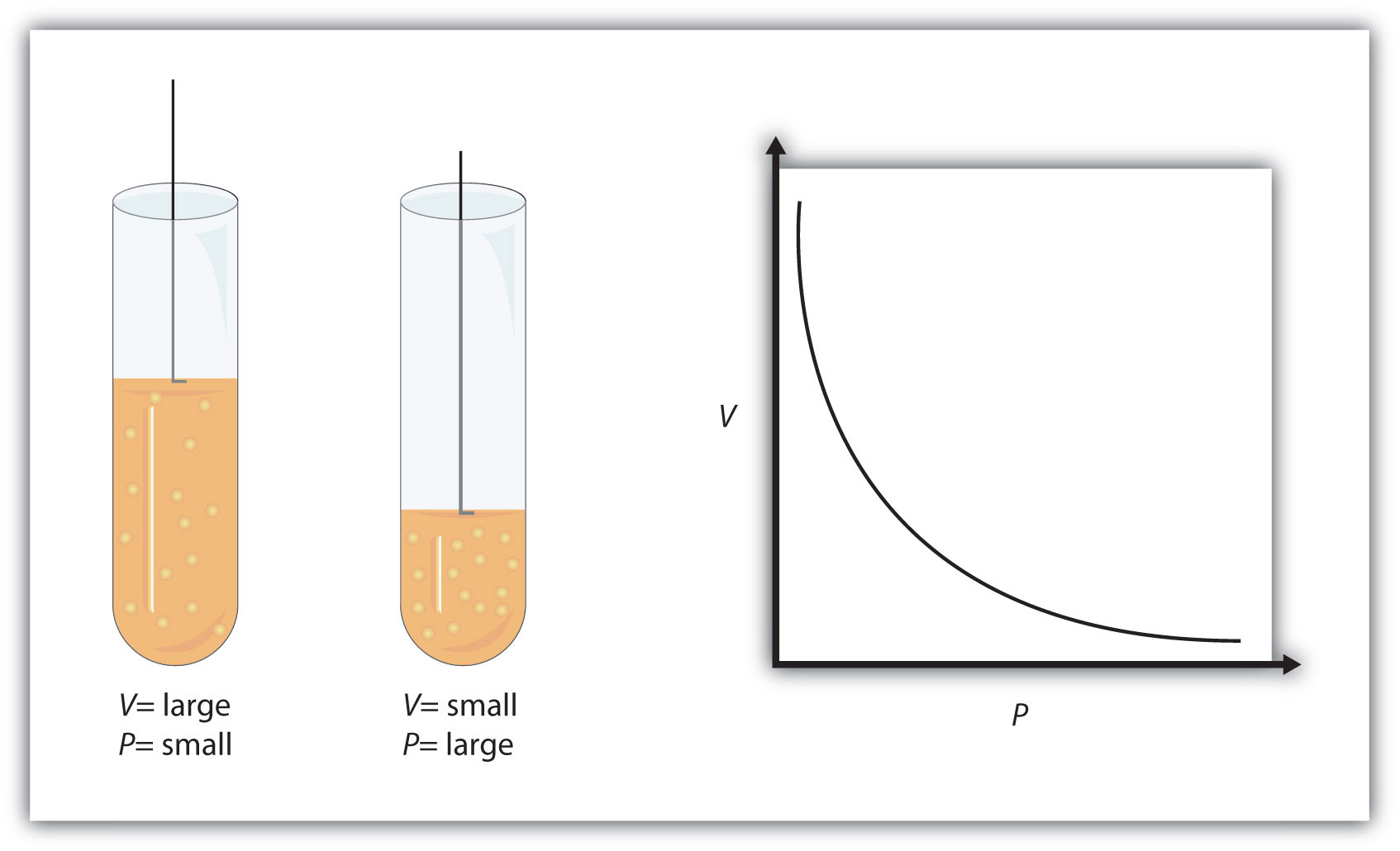
In your case, the pressure is increasing, which can only mean that the volume must decrease.
Now, the inverse relationship that exists between pressure and volume is given by the equation
#color(purple)(bar(ul(|color(white)(a/a)color(black)(P_1 * V_1 = P_2 * V_2)color(white)(a/a)|)))#
Here
Rearrange to solve for
#P_1 * V_1 = P_2 * V_2 implies V_2 = P_1/P_2 * V_1#
Plug in your values to find
#V_2 = (1.50 color(red)(cancel(color(black)("atm"))))/(4.50color(red)(cancel(color(black)("atm")))) * "75.0 mL" = color(green)(bar(ul(|color(white)(a/a)color(black)("25.0 mL")color(white)(a/a)|)))#
The answer is rounded to three sig figs.
As you can see, because the pressure increased by a factor of

