What is degeneracy as opposed to a degenerate state? How can we know what orbitals are higher in energy?
1 Answer
This is a kind of complicated question.
- A degenerate state is a state in which the energy is the same as other states.
- A degeneracy is the number of states that have that same energy, and is described as
#2l+1# .
So, the difference is that degeneracy describes how many states, and a degenerate state is specifically which ones count.
- Any
#ns# orbital is the same energy for the same#n# . - No,
#p# and#d# orbitals of the same#n# don't necessarily have the same energy (not even in hydrogen atom). - I cannot tell you the energy of every orbital, because their energies change throughout the periodic table, and sometimes the actual ordering is different.
DEGENERATE STATES
Atomic orbitals that share the same principal quantum number
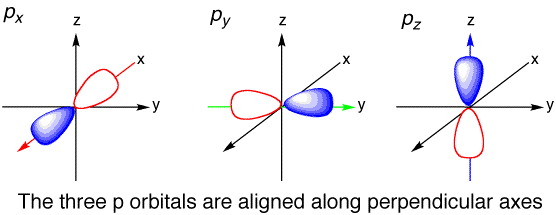
(if they share the same
On the other hand, if their
For example, the
- They do not have the same shape (different
#l# ). - They do not have the same total number of radial nodes (different
#n - l - 1# ). - They do not have the same number of angular (planar) nodes (different
#l# ). - Maybe some combination of all three.
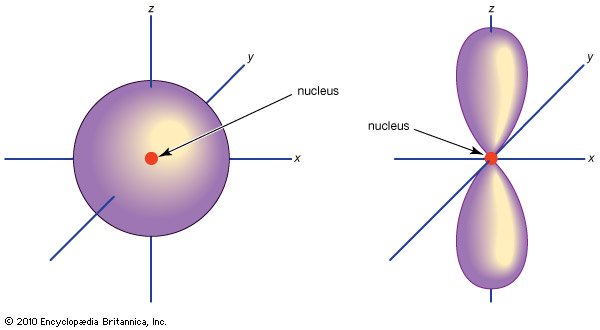
However, the
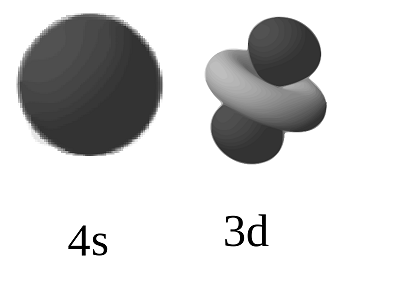
One might call them degenerate once the energies get close enough. When comparing their
DEGENERACY
Like I said, degeneracy is just the number of orbitals of the same energy. Typically we say, for example:
- The
#np# atomic orbitals are triply degenerate, because there are three of them in a free atom, and they are all equivalent orbitals with equivalent energies. - The
#np# atomic orbitals have a degeneracy of#2l + 1 = 3# , since their#l# is equal to#1# .
For example, a
#ul(uarr darr) " " ul(uarr color(white)(darr)) " " ul(uarr color(white)(darr))#
#underbrace(" "" "" "" "" "" "" "" ")#
#2p_x" "" "2p_y" "" "2p_z#

