What type of bond is formed in a diatomic element where the two atoms are identical?
1 Answer
Oct 20, 2016
It would be a non-polar covalent bond.
Explanation:
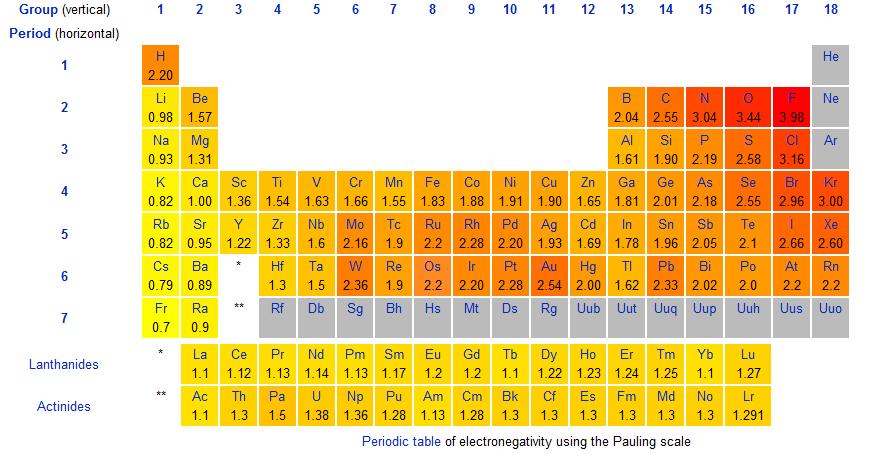
The difference in electronegativity determines the bond type between atoms. If the difference is 0 to 0.4, it is considered non-polar covalent. Electronegativity is the tendency of an atom to attract bonded electrons to itself. The stronger the tendency, the higher the electronegativity.
A diatomic element in which both atoms are the same, such as
The following chart gives the electronegativities of the elements.