What is the molecular geometry of #ICl_5#?
1 Answer
Square pyramidal
Explanation:
The molecular structure of
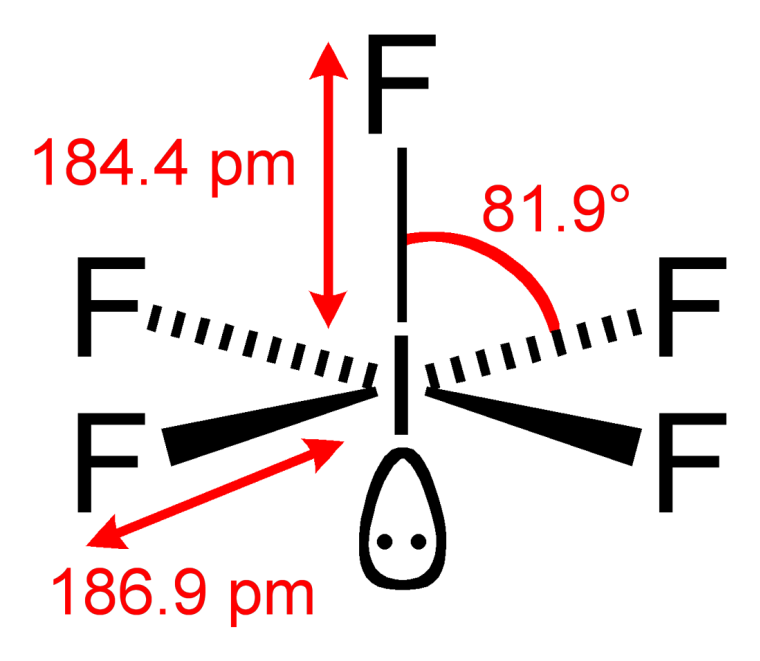
If the lone pair of electrons was another atom, the geometry would be octahedral. Because of VSEPR theory, the paired electrons repel the other atoms more than an atom would, giving it a different shape. In this case, the geometry is square pyramidal.


