How do you determine if a molecule has a symmetrical shape?
1 Answer
See explanation.
Explanation:
You must first determine its VSEPR Shape (tetrahedral, pyramidal, linear, etc.).
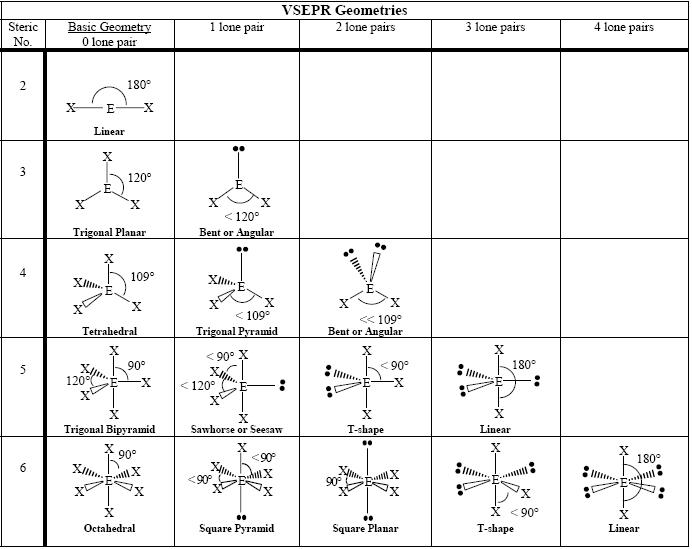
After drawing it out, look at the surrounding atoms of the central atom. If the surrounding atoms are all the same, then the dipoles cancel out, thus the molecule has a non-polar shape (which is non-polar in general - but there are also covalent bonds to consider - where you look at electronegativity).
Some examples:
- Methane
#(CH_"4")# has an EN of 0.4 - non-polar covalent bond. It's also symmetrical thus it is a non-polar molecule (in general). - Boron trichloride
#(BCl_"3")# has an EN of 1 - polar covalent bond. However, it is symmetrical, thus it is non-polar in general but it has a polar covalent bond.
Hope this helps :)


