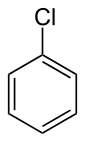
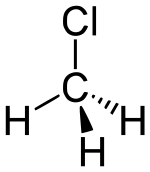
Why is the #"C"-"Cl"# bond length in chlorobenzene shorter than in methyl chloride?
1 Answer
From the NIST database, the
For perspective, the average bond length is about
DISCLAIMER: It's such a small difference in bond length that I think you can take this explanation with a grain of salt. What matters is the reasoning, really.
SPATIAL ARGUMENT
- The atom group bonded to the
#"Cl"# is a#"C"-"C"-"C"# on benzene, and the group bonded on methyl chloride is#"CH"_3# .
The larger size of the group may increase the bond length, mainly due to spatial arguments. But this is probably minor.
ELECTRON DENSITY DISTRIBUTION + DIPOLE MOMENT
The dipole moments should be considered for sure.
- Benzene is electron-withdrawing, but so is
#bb("Cl")# . So, electron density is held closely to both the#"C"# and#"Cl"# atoms. You could also say that the electron density is delocalized throughout benzene, decreasing the dipole moment of the molecule.
The dipole moment is
#bb1.690# .This results in a shorter required bond length for the most optimal equilibrium bond distance, since the electron density is more evenly distributed between
#"C"# and#"Cl"# .
#"CH"_3# is electron-donating, and that works together with#"Cl"# 's inductive electron-withdrawal to polarize the electron density towards chlorine (compare with previous reason about even electron density distribution).
This bond polarization lengthens the bond due to the increased ionic character of the bonding (as a result of the lesser electron sharing). I say that because weaker bonds are generally longer.
The dipole moment is
#bb1.870# .
I would think that it's those three effects that all occur at the same time, giving a small difference in bond length between chlorobenzene and methyl chloride...
It's complicated, but I think that covers most of it. Mainly, some contribution of each of the above three reasons cover why the bond lengths are how they are, and it's not entirely clear how significant each contribution is.

