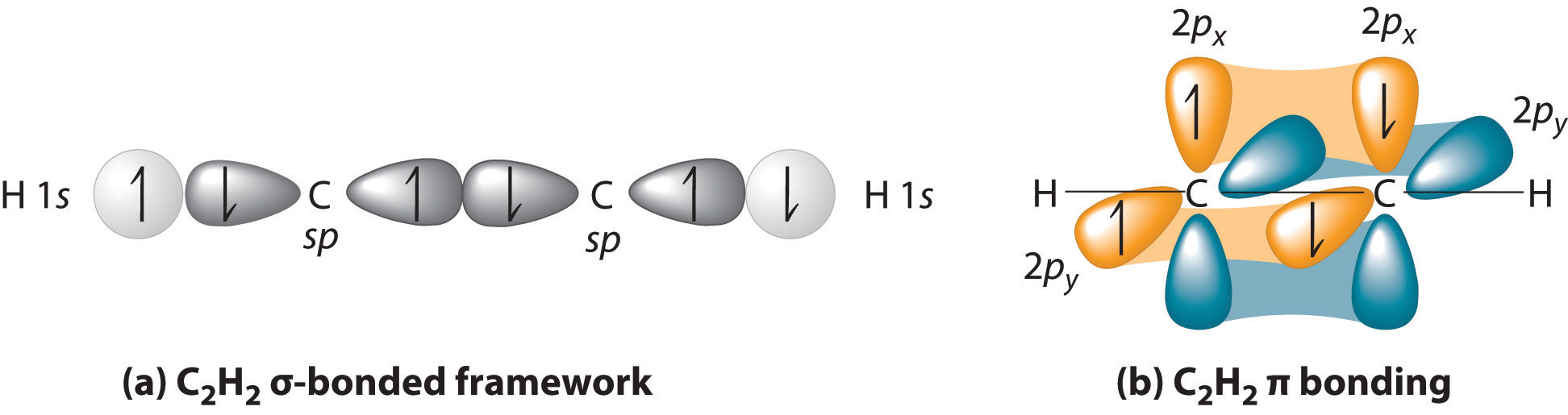
Carbon uses #sp# orbitals in this compound for its #sigma# bonds, and #2p_x# and #2p_y# atomic orbitals in its #pi# bonds. Only the #2s# and #2p_z# are hybridized to form the two #sp#-hybridized atomic orbitals on #"C"#.
Silver acetylide can be thought of as analogous to acetylene, #"C"_2"H"_2#, since the valence orbitals on carbon do not change whether it is #"Ag"^(+)# or #"H"^(+)# bonding to #"C"#.
#stackrel((+))("Ag") :stackrel((-))("C"):::"C":"H"#
or
#stackrel((+))("Ag")-stackrel((-))("C")-="C"-"H"#
Choosing the right #"C"-"H"# bond for simplicity, hydrogen can only contribute a #1s# atomic orbital in a linear combination to overlap with carbon's atomic orbital in a #sigma#/sigma fashion (head-on overlap).
Here are examples of #bb(sigma)# (head-on) and #bb(pi)# (sidelong) overlap:
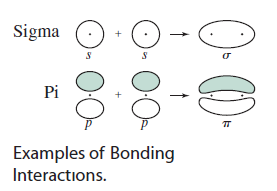
Note that the same-signed lobes overlap to create a bonding molecular orbital.
Although carbon could offer its #2p_z# atomic orbital, it also needs to bond to the other carbon atom. It's more favorable if both orbital lobes are the same sign, but originally, a #2p_z# atomic orbital has oppositely-signed lobes.
So, a regular #2p_z# is not sufficient.
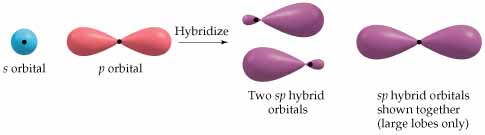
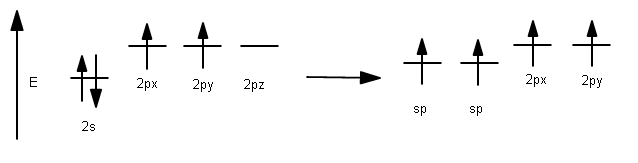
As a result, each carbon hybridizes its #ul(2p_z)# atomic orbital with its #ul(2s)# atomic orbital to form two #bb(sp)#-hybridized atomic orbitals (and we should expect from conservation of orbitals that we get two hybridized orbitals out from two pure orbitals in).
Orbital depiction:
Electron configuration depiction:
This allows both carbons to bond with both each other and hydrogen and silver in silver acetylide. Here is an MO depiction for acetylene for reference:
By analogy, silver can bond with carbon's #sp#-hybridized atomic orbital by overlapping its #\mathbf(5s)# atomic orbital (just like how hydrogen uses its #1s# atomic orbital), since it is singly-occupied in the configuration #[Kr] 5s^1 4d^10# as an "exception" to the Aufbau Principle.
The only difference between acetylene and silver acetylide is that silver contributes a different orbital in the linear combination. Carbon contributes the same orbital as with hydrogen.




