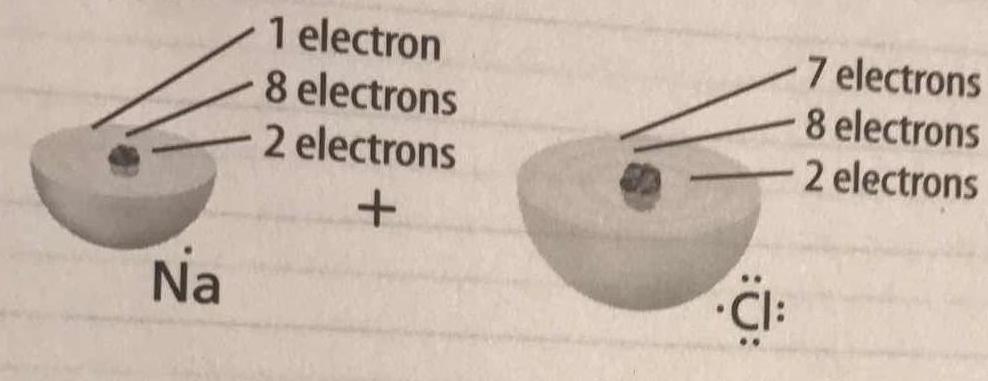
In the image below, if sodium loses one electron and chlorine gains one electron, how many valance electrons do sodium and chlorine now have?
1 Answer
Dec 4, 2016
Both will have eight valence electrons.
Explanation:
The single valence electron in the third energy level of the sodium atom is transferred to the valence shell in the third energy level of the chlorine atom.
The sodium atom now has eight valence electrons in its second energy level. Since it lost one electron, it is now a sodium ion with a charge of
The chlorine atom now has eight valence electrons in its third energy level, having gained the electron from the sodium atom. Since it gained one electron, it is now a chloride ion with a charge of
The electrostatic attraction between the oppositely charged ions forms the ionic bond


