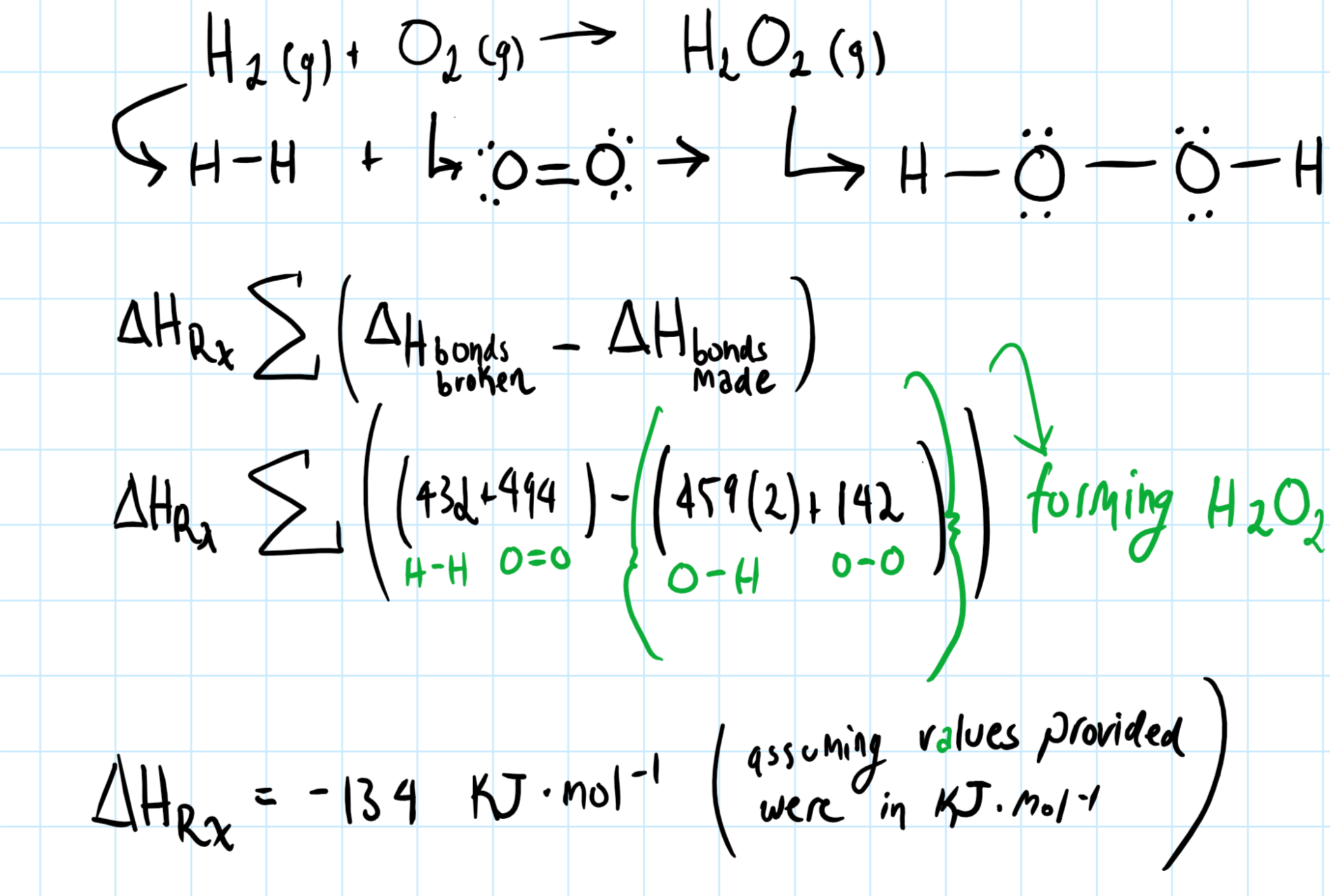How do you determine the heat of formation of hydrogen peroxide from the given bond energies: #H_2(g) + O_2(g) -> H_2O_2(g)#? Bond energies: H-H = 432; O2 = 494, O-H = 459. O-O = 142?
1 Answer
Dec 18, 2016
See below
Explanation:
I'd maybe draw out the lewis structure for the reacting species, this is important in figuring the
For the
The specific energies are shown in parenthesis.