Where does the positive charge come from on an atom?
1 Answer
Dec 19, 2016
If you're talking about just atoms after an ionization process, then cations.
By definition, cations have a positive charge, since they've lost an electron, which itself has a negative charge:
#"M" -> "M"^(+) + e^(-)#
By losing a negative charge, the atom has gained a positive charge.
If you're wondering about what part of the atom... the nucleus is where the positive charge resides. Here is a (simplistic) depiction of the atom, showing the positive charge in the nucleus:
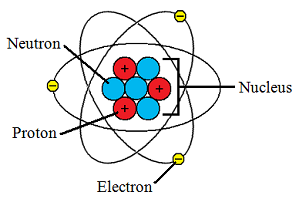
The protons in the nucleus (red) have the positive charge, and such a positive charge arises from the imbalance of protons and electrons - specifically, when the number of protons is greater.

