Question #30be5
1 Answer
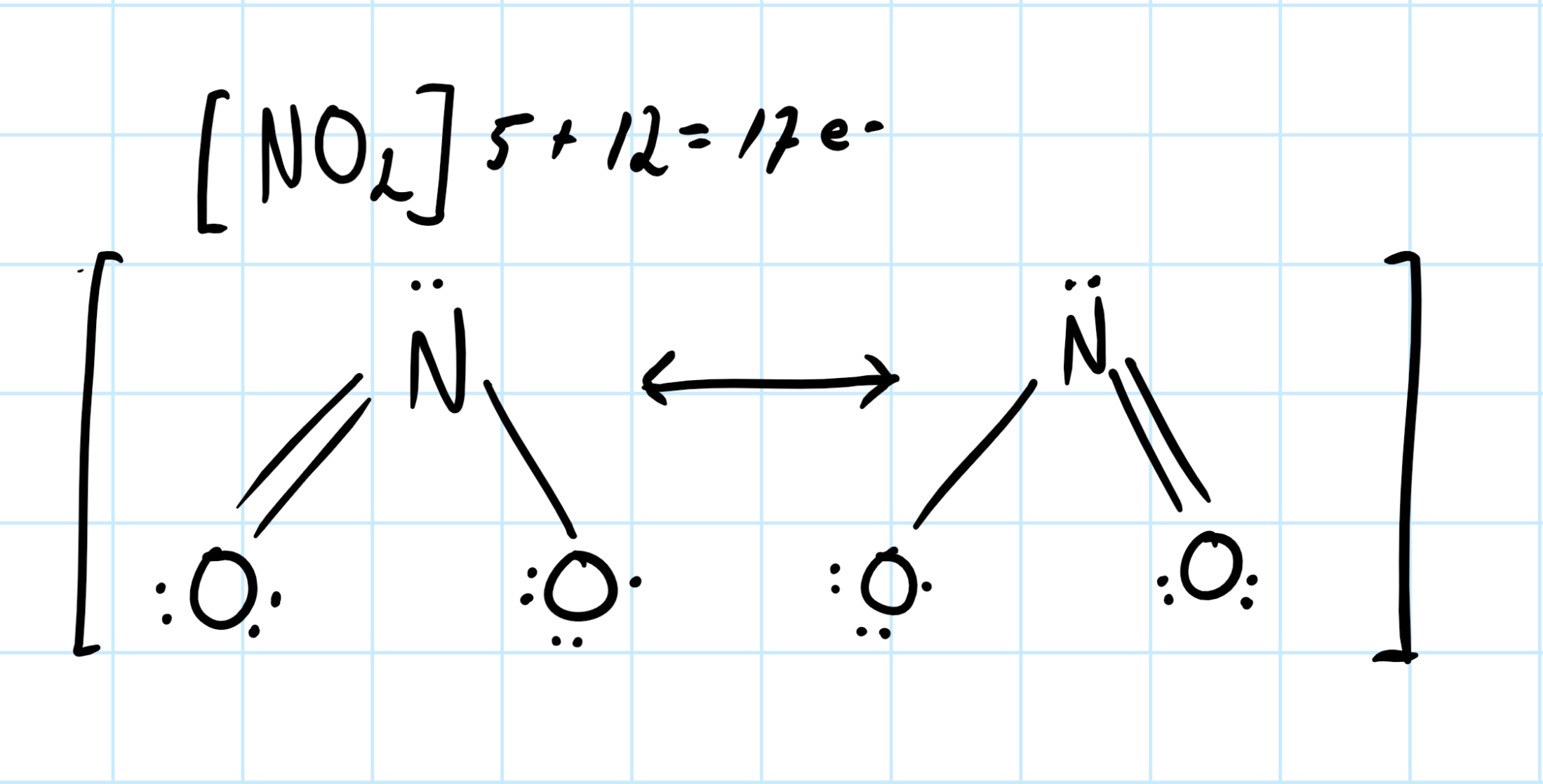
3 shared pairs in
(you gave a whole chemical equation though and not a specific molecule, so I don't know which molecule to specify)
Explanation:
So for nitrogen dioxide, I would say the number of shared pairs is three. Consider the lewis structure of species. Lone pairs about the atoms are not shared, and electron involved in bonding are shared about the species.
Bonding is completely driven by the electronic properties of the species.
I'd argue the correct way to draw a lewis structure of
In the single bond one pair is shared, and in the double bond there are two pairs of electron being shared. So this means that there are three shared pair total.
Consider the graphic below: