How do you find the geometry of #TeCl_4# using the VSEPR method?
1 Answer
Draw the lewis structure to find the electronic geometry and use it to determine the molecular geometry.
Explanation:
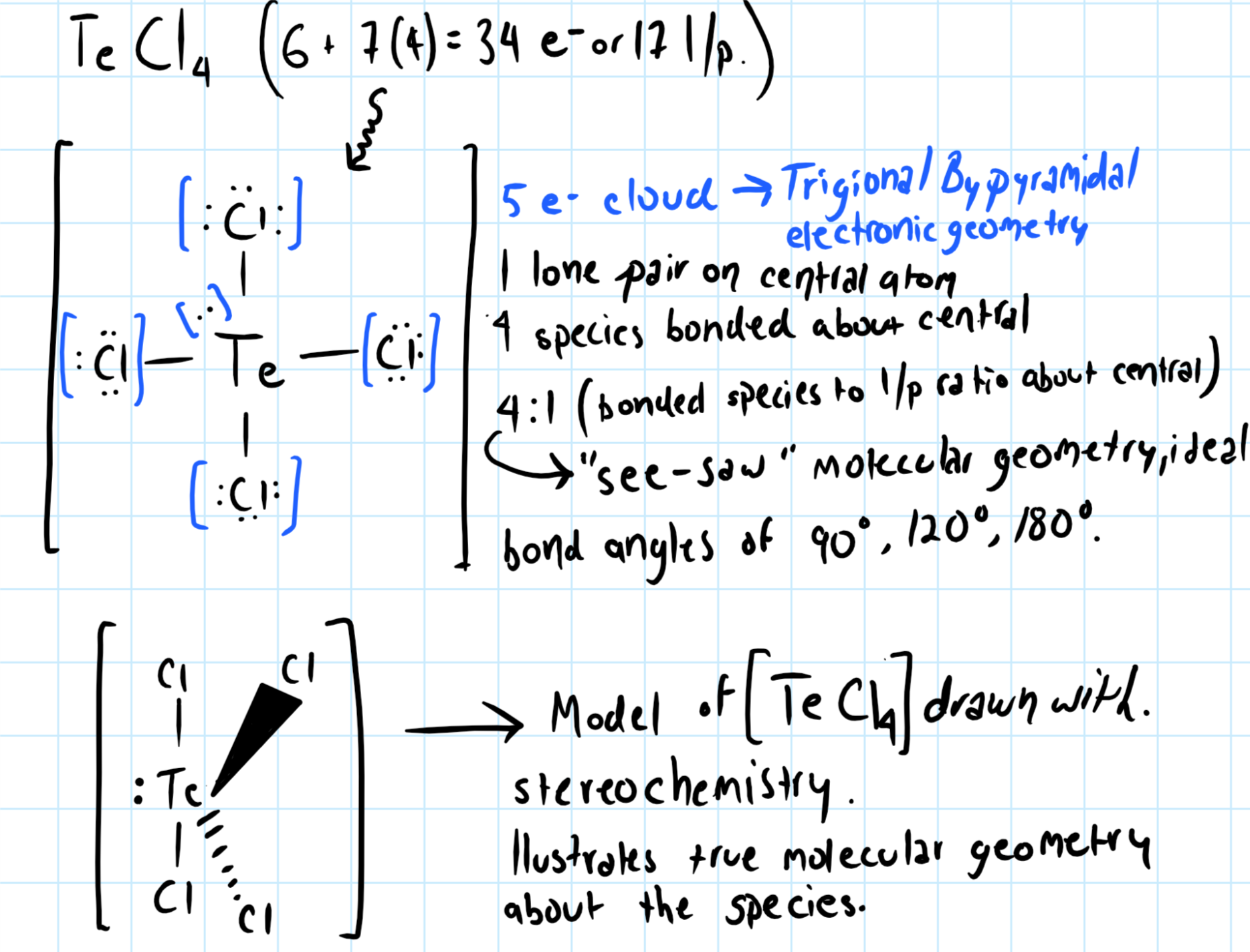
For
With 5 electron cloud about the central species,
To determine the molecular geometry, we consider the ratio of bonded species to lone pair about the central atom.
There are five electron clouds around the central atom, and the ratio of bonded species to lone pair is 4:1, so the
"see-saw" molecular geometry, with ideal bond angles of
However, the lone pair of electron about the
This means the actual bond angles for
Consider the graphic below: