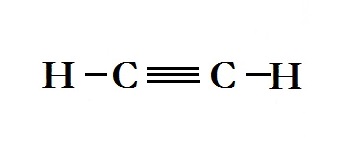What kind of bond is there between carbons in #H_2C_2#?
1 Answer
Jan 9, 2017
The bond between the carbon atoms in the compound
Explanation:
The diagram below represents the structural formula for