Explain the mixture separation techniques?
2 Answers
From this website, I'm finding common separation techniques to be:
- Paper chromatography
- Filtration
- Evaporation
- Simple distillation
- Fractional distillation
- Magnetism
- Separating funnel
I realize that that is a lot, so if you are looking for specific techniques and not all of these, just scroll through and find what you need.
I added big headings to make it easier to find what you need.
DISCLAIMER: LONG ANSWER!
PAPER CHROMATOGRAPHY
A basic setup can look like this:
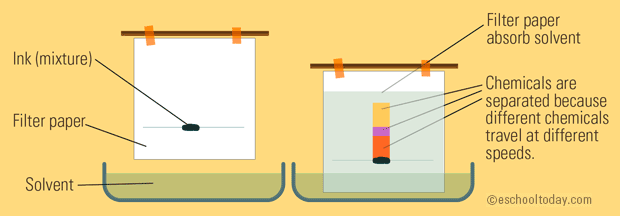
What you have is a container with a liquid solvent in it; this solvent is may or may not have similar properties (polarity; a defined positive and negative end of a molecule) with the mixture blotted onto the paper.
The solvent travels up the paper.
- The more similar the polarity of the chemicals in the mixture and that of the solvent, the more quickly they travel up the paper together because they are attracted to each other well.
- The more different the polarity of the chemicals and that of the solvent, the more slowly they travel up the paper together because they are not attracted to each other well.
So, you get different chemicals traveling at different speeds up the paper, separating them from each other in the mixture.
FILTRATION
Basically, filtration is taking advantage of the size of the particles in a mixture to separate them.
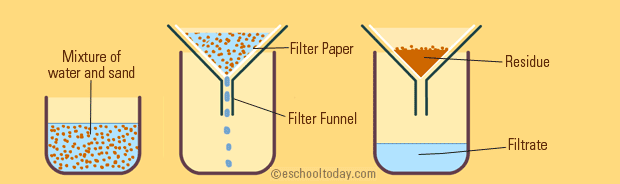
The setup you see above is a sand/water mixture, and all that's happening is that it's put through filter paper (such as a coffee filter).
The filter paper has small pores that are smaller than the sand itself, so the sand gets stuck on one side of the filter paper while the water drips through because water molecules are small enough to get through.
So, your sand (residue) and water (filtrate) are separated by their size.
EVAPORATION

This is actually not that complicated; if you have a mixture of water and salt, and you heat it a lot, the water will eventually reach its boiling point, the temperature where it wants to become a gas.
So, the water vaporizes, and floats up into the air, leaving the salt by itself. With that, you've isolated the salt by removing the water.
SIMPLE DISTILLATION
This is a little more complicated, but basically built off of evaporation. There is still heating involved and your water is still vaporizing.
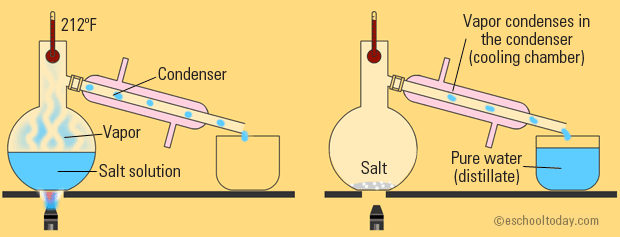
The only difference is that now, you've attached a condenser tube that is surrounded by cold liquid. This cold liquid forces the water vapor to condense back down to a liquid and travel into a new container.
So, basically, simple distillation is just evaporation with a way to collect your water instead of letting it float up into the air.
FRACTIONAL DISTILLATION
This can get complicated, but it's a similar principle to "simple distillation".
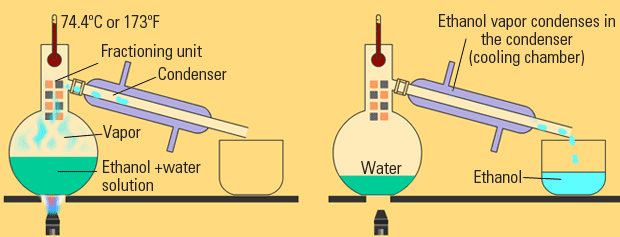
The main difference between simple and fractional distillation is that now you're dealing with two liquids instead of a solid in a liquid.
With two liquids, which are generally similar in feel, it's more delicate of a process, so it takes longer.
In this case, two different liquids might have different boiling points, which is the idea used here. It means one will become a gas more easily than the other.
The basic principles found in distillation are the same; it's basically special evaporation with a way to collect the vaporized liquid.
MAGNETISM
Here, you would have to know ahead of time whether one substance will respond to a magnet or not.

For example, iron will get attracted to a magnet, but sand will not, so you can pull the iron out and leave the sand back in the container.
SEPARATING FUNNEL
This is pretty much the same as filtration, with no filter paper. You can take two liquids of different densities (how much mass is in a given volume) and pour them into a funnel. An example is oil and water.
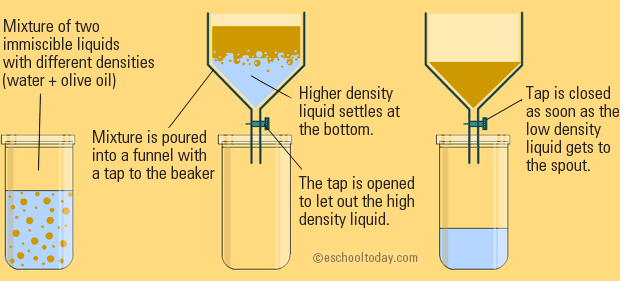
When the mixture settles, the denser liquid will be at the bottom, and drips through the funnel first.
This is a separation that you can just let occur naturally.
It might be useful to classify separations by the mechanisms first, then the method.
Explanation:
Compounds can be separated on the basis of density, physical phase, and chemical affinity – what things tend to stay together due to their chemical structure and properties.
Filtration separates solids from liquids. This is a physical separation based on density.
Evaporation is really just creating the conditions for solid precipitation and removal. It may be considered and alternative to filtration, or used when the maximum retention of the solid is desired.
Whether “simple” or “fractional”, distillation is really separation by density primarily. It is directly driven by differences in boiling points, which will not always follow density if other chemical affinity factors are involved. It separates liquids from each other, also leaving solids behind as in evaporation, if any are present.
A separatory funnel is also a liquid-liquid separation mechanism that depends primarily on density and/or chemical affinity. That is, immiscible compounds to not want to remain together, so ionic and covalent compounds can be separated. In any case, it is the most dense fluid that is removed by separation from the bottom of the funnel.
Decantation may be considered a separation method in this regard as well, as it can be used to separate a lighter, top level of liquid from a heavier, lower one. It can also be used instead of filtration to remove liquid from a denser solid.
Magnetism is not really a “common” method of separation in chemistry, but it can be useful in separating magnetic from non-magnetic compounds. This does not address the issue of heterogeneous metal mixtures in both the magnetic and non-magnetic parts of the original mix.
Chromatography is used in several forms – Paper (or more properly, fixed substrate), liquid-liquid, and gas-liquid – depending on the types of compounds desired to be separated. The mechanism of chromatography however is based on chemical affinity and solubility. Thus, it is an application of separation mechanisms, but not a “fundamental” separation mechanism itself.
One not previously mentioned, but widely used is centrifugation – where solid-liquid, liquid-liquid, and even gas-gas mixtures are separated on the basis of density like a separatory funnel by rapid spinning to create a strong gravitational gradient in the mixture.


