What is the basic unit of an ionic compound and a covalent compound?
1 Answer
Jan 17, 2017
The basic unit of an ionic compound is called a formula unit. The basic unit of a covalent compound is a molecule.
Explanation:
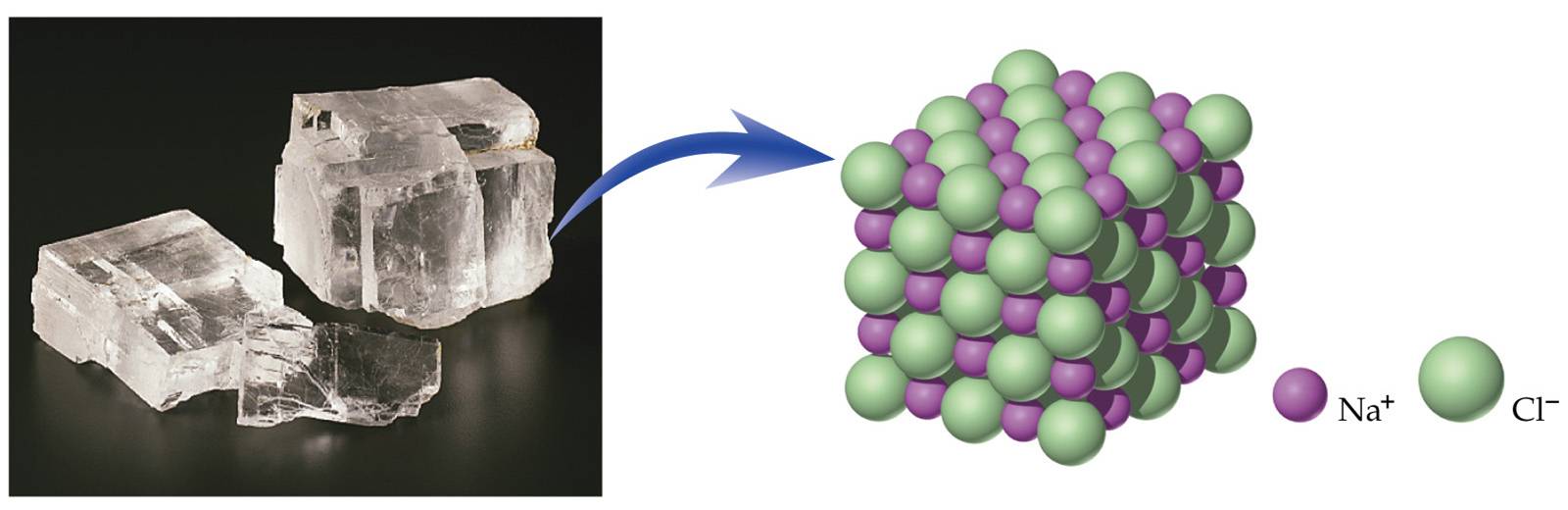
Ionic compounds exist as ionic lattices containing large numbers of positive and negative ions. The formula unit is the lowest whole-number ratio of positive to negative ions.
Halite crystals are composed of huge numbers of sodium and chloride ions arranged in a three-dimensional lattice.
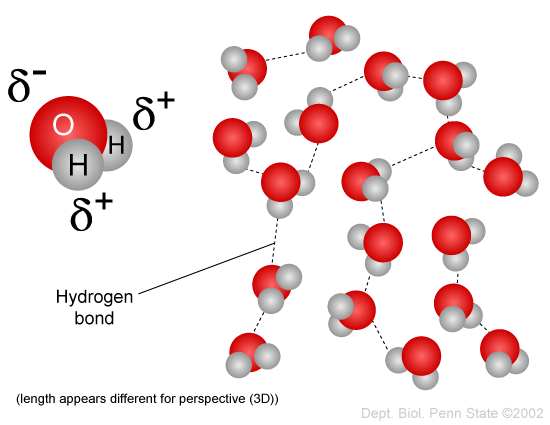
Covalent compounds are composed of individual molecules. An example is the polar molecule water. The symbols