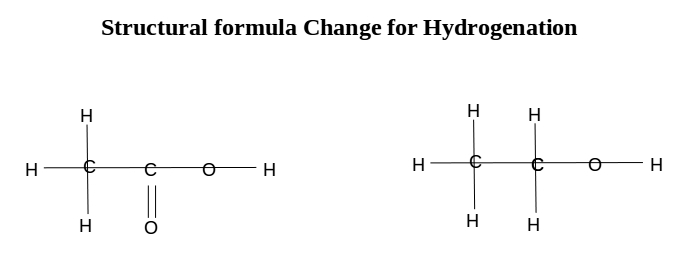
You use the oxidation numbers of #"O"# and #"H"# to figure out the oxidation numbers of #"C"#.
#stackrelcolor(blue)(0)("C")_2 stackrelcolor(blue)("+1")("H")_4 stackrelcolor(blue)("-2")("O")_2 + stackrelcolor(blue)(0)("H")_2 → stackrelcolor(blue)("-2")("C")_2 stackrelcolor(blue)("+1")("H")_6 stackrelcolor(blue)("-2")("O")+ stackrelcolor(blue)("+1")("H")_2stackrelcolor(blue)("-2")("O")#
#stackrelcolor(blue)(0)(color(white)("C"))color(white)(l)stackrelcolor(blue)("+4")(color(white)("H"))color(white)(ll)stackrelcolor(blue)("-4")(color(white)("O"))color(white)(mmmmm)stackrelcolor(blue)("-4")(color(white)("C"))color(white)(l)stackrelcolor(blue)("+6")(color(white)("H"))color(white)(ll)stackrelcolor(blue)("-2")(color(white)("O"))color(white)(ml)stackrelcolor(blue)("+2")(color(white)("H"))color(white)(ll)stackrelcolor(blue)("-2")(color(white)("O"))#
You write the oxidation numbers of #"O"# and #"H"# above the atoms and their total oxidation number beneath them.
Thus, for #"C"_2"H"_4"O"_2#, the sum of their oxidation numbers is #+4-4 = 0#.
Hence, the sum of the oxidation numbers of the two #"C"# atoms is zero, so their average oxidation number is zero.
Similarly, in #"C"_2"H"_6"O"#, the sum of the oxidation numbers of #"O"# and #"H"# is #+6-2 = +4#, so the two #"C"# atoms must add up to -4, or -2 for each #"C"# atom.
We see that the oxidation number of #"C"# decreases from 0 to -2, so the #"C"# atoms are reduced.
Similarly, the oxidation number of #"H"# increases from 0 in #"H"_2# to +1 in water, so the hydrogen is oxidized.