How do you determine alpha decay?
1 Answer
First know what is an alpha particle
Alpha decay is the decomposition of a nucleus of an element into a new nucleus of a different element and an alpha particle. Alpha particle is composed of 2 neutrons and 2 protons. Generally an alpha particle is referred to a helium nucleus since it has 2 neutrons and 2 protons but no electrons.
Charge of an
2protons =
2neutrons =
Therefore an alpha particle has a charge of
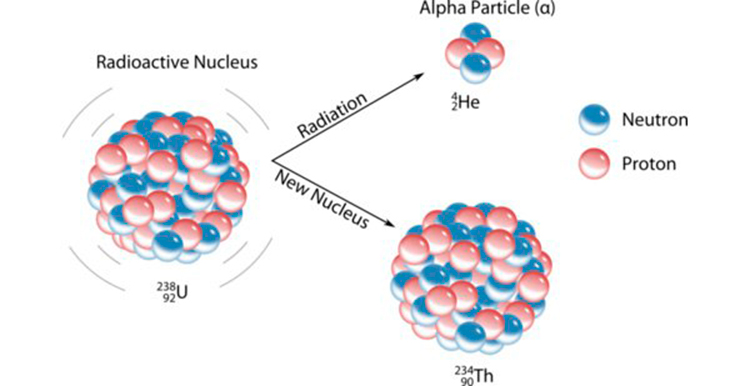
This photo indicates the alpha decay of uranium
The new nucleus that is formed which is Thorium is called the daughter nucleus and the nucleus which is decaying is the father nucleus which is uranium .
Now lets cone to the main question.
How to calculate the products of an alpha decay
First step In every alpha decay an alpha particle is formed though all alpha decay have different daughter nucleus . So first look at the father nucleus and list its number of protons and its atomic weight.
Its easy to understand when we solve an example . So lets take metal
Step 2) Calculate the number of neutrons from the following equation
14 - 6protons = 8neutrons
Step 3) Now from number of neutrons subtract 2 and from number of protons subtract 2 as an alpha particle has 2 neutrons and 2 protons and in an alpha decay an alpha particle will always form in case of any any father nucleus.
For example (Please note that this is only an example)
protons = 6 so 6-2 = 4
neutrons= 8 so 8 -2 = 6
Step 3) After subtracting add the remaining protons and neutrons (4+6 = 10) 10 is the atomic weight of the new element nucleus.
So now this alpha decay can be represented as
(You can also solve the equation by directly subtracting 4 from the atomic weight and 2 from number of protons but in chemistry we cannot give brief answers !!! )
If it were a real example you can check the element which has an atomic mass of 10. But as this is an example there is no element with 10 as the atomic weight
y metal is the new element nucleus formed.
Now lets solve a real alpha decay equation
Represent the alpha decay of Uranium
Atomic mass of seaborgium = 263
Protons = 106
neutrons = 263 - 106 = 157
106 - 2 = 104 protons
157- 2 = 155 neutrons
104 + 155 = 259
The element which has 259 as the atomic weight is rutherfordium.
So the equation is

